Tissue Engineering, Inflammation & phytochemicals
Research Group Professor Dr. M. Shakibaei
Musculoskeletal- & Tumor Biology Research Group
Team members:
Univ. Prof. Dr. Mehdi Shakibaei (Research Group Leader)
LUDWIG-MAXIMILIANS-UNIVERSITY MUNICH,
Faculty of Medicine, Institute of Anatomy,
Pettenkoferstrasse 11, D-80336 Munich, Germany.
Phone: (089) 2180 72 624
Fax.: (089) 2180 72 625
E-Mail: mehdi.shakibaei@med.uni-muenchen.de
PD Dr. med. vet. Constanze Buhrmann (Postdoctoral fellow)
Phone: (089) 2180 72 626 or 72 674
E-Mail: constanze.buhrmann@med.uni-muenchen.de
Mrs. Christine Meyer (Scientific officer)
Phone: (089) 2180 72 626 or 72 674
E-Mail: christine.meyer@med.uni-muenchen.de
Mrs. Aranka Brockmüller (Doctoral candidate and Technician)
Phone: (089) 2180 72 674, 72 671
E-Mail: aranka.brockmueller@med.uni-muenchen.de
Papers: Publication list
Current Research Collaborations:
Prof. Dr. med. Ralf Stahlmann: Institute of Clinical Pharmacology and Toxicology, UKBF, Charite Universitätsmedizin Berlin, Germany.
Prof. Dr. Michael Schäfer: Department of Anesthesiology and Intensive Care Medicine, Charité - Universitätsmedizin Berlin, Germany.
Dr. Andreas Eimannsberger: Institute of Anatomy, Ludwig-Maximilians-University Munich, Germany.
Dr. Wolfgang Lorenz: Institute für Innenraumdiagnostik, Düsseldorf, Germany.
Prof. Dr. med. Bharat Aggarwal: Cytokine Research Section, Dep.of Molecular Oncology, University of Texas M.D. Anderson Cancer Center Houston, USA.
Dr. Ajay Goel: Gastrointestinal Cancer Research Laboratory, Division of Gastroenterology, Baylor Research Institute and Charles A. Sammons Cancer Center, Baylor University Medical Center, Dallas, USA.
Prof. Dr. Parviz Shayan: Department of Parasitology, Faculty of Veterinary Medicine, University of Tehran, Iran.
Prof. Dr. Ali Mobasheri: School of Veterinary Medicine and Science, University of Nottingham, UK.
Prof. Dr. Ajaikumar Kunnumakkara: Cancer Biology Laboratory, Indian Institute of Technology, Guwahati, India.
Prof. Dr. Peter Kubatka: Department of Medical Biology; Jessenius Faculty of Medicine, Comenius University Bratislava, Slovakia.
Prof. Dr. Dietrich Büsselberg: Weill Cornell Medicine, Doha, Qatar
Main Research Topics:
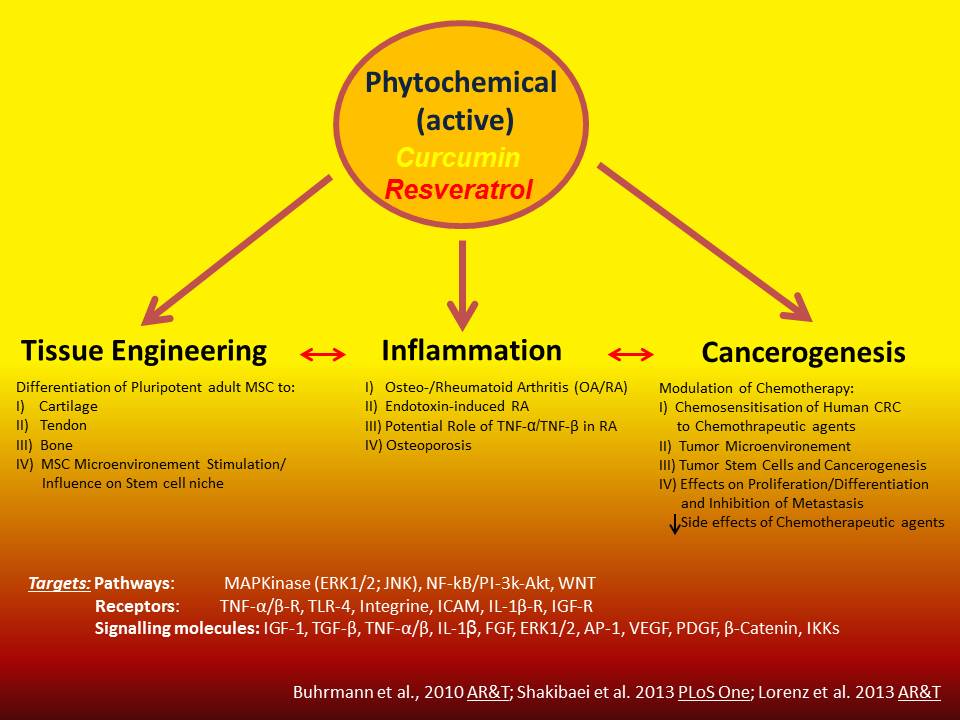
Project 1: Tissue Engineering, Inflammation & phytochemicals
A: Stem cells
B: Cartilage & phytochemicals
Due to their pluripotent differentiation potential MSCs offer themselves as an attractive cell source for in vitro production of transplantable tissue. The aim of this project is to investigate the influence of primary tissue cells on the differentiation potential of MSCs in vitro. To this end, in our studies we have co-cultivated MSCs with different primary tissue cells such as osteoblasts, chondrocytes and tenocytes. We explore various mixing ratios, cultivation systems (monolayer versus high density mass culture/ alginate culture) and the influence of specific growth factors. Evaluation of these results is carried out by means of light microscopy, immunofluorescence, electron microscopy, immune electron microscopy and western blot analysis.
Presentation of projects:
1A: Isolation and characterisation of mesenchymal stem cells
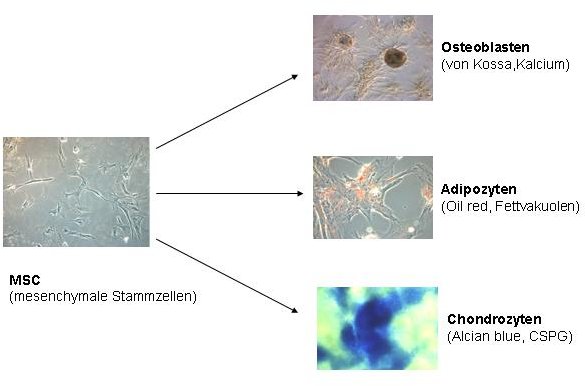
To attest their pluripotency, we differentiate the obtained mesenchymal stem cells (MSCs) by means of various growth factors and phytochemicals towards osteoblasts, adipocytes and chondrocytes. Our group successfully isolates human, canine and ovine mesenchymal stem cells (MSCs) from bone marrow and adipose tissue. For this purpose, diluted bone marrow aspirates are layered over a density medium, centrifuged and the obtained cells cultured in vitro. Similarly, adipose tissue is digested with collagenases, and the obtained cells cultured in vitro. In both cases, a few cells can attach themselves and grow into colonies. Prior to further use in our studies stem cell characterization of these cells is performed on the basis of surface markers and their differentiation potential. MSCs possess a number of specific surface receptors, for example CD 105+ and CD90+. Generally MSCs do not express hematopoietic stem cell surface receptors such as CD34 and CD45. Additionally, MSCs have multilineage differentiation potential and can be successfully differentiated into osteoblasts, chondrocytes and adipocytes in our group. Evaluation of these results is carried out by means of light microscopy, immunofluorescence, electron microscopy, immune electron microscopy and western blot analysis.
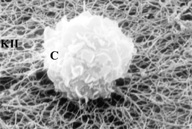
Scanning electron micrograph of chondrocyte (C). The chondrocyte growing on collagen type II (KII), takes on a round shape from the beginning of the cultivation period.
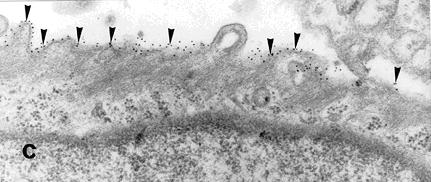
Immuno-morphological studies with electron microscopy, with gold-conjugated secondary antibodies directed against primary integrin antibody, show that β1-integrins are expressed on the surface of chondrocytes.
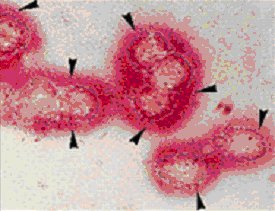
Immunodetection of collagen type II by APAAP method
Die Kultivation von isolierten Chondrozyten auf Kollagen Typ II führt zur Stabilisierung des Chondrozyten-Phänotyps. Chondrozyten überleben in Anwesenheit von knorpel- spezifischer Matrix in der Kultur länger und halten ihren Phänotyp aufrecht, mit großer Wahrscheinlichkeit durch Vermittlung der ß1-Integrine
1B: Osteoarthrosis/osteoarthritis and phytochemicals
Osteoarthrosis/osteoarthritis (OA), the most common disease of the joints, is defined as a chronic progressive degenerative joint disease in which the exact causes are still largely unknown. OA leads to the degradation of extracellular matrix (ECM) proteins in the cartilage mainly by proteases (particularly matrix metalloproteinases). Assembly and disassembly of the ECM proteins are normally in equilibrium, which is regulated by cytokines and growth factors. During OA this finely tuned interplay is changed and there is an increased degradation of extracellular matrix proteins leading to cartilage and subchondral bone degradation and deformation. The aim of the project is on one hand to investigate and to characterize in vitro those pro-inflammatory cytokines that may play a central role during OA (eg, TNF-alpha, IL-1beta) and on the other hand to investigate and characterise growth factors and polyphenols which may counteract the catabolic effects induced by pro-inflammatory cytokines in chondrocytes.
Therapeutic inhibition of pro-inflammatory cytokins by phytochemicals in
the cartilage is currently a promising approach in the etiological treatment of osteoarthritis. The detailed elucidation of specific targets and cytokine-induced signal transduction pathways would greatly improve this osteoarthritis therapy approach. Therefore, in various projects therapeutically useful natural polyphenols (phytochemicals such as curcumin, resveratrol, nettle leaf extract, devil's claw root extract, etc.) are examined for inhibition of pro-inflammatory cytokine-induced signaling pathways.
Methods:
1: 2D and 3D culture models (Monolayer-, High Density- & Alginate Cultures),
2: 3D culture model mimicking the metastatic tumor microenvironment in vivo,
3: Morphology (Light- and Electron microscopy),
4: Immunmorphology (Immunfluoresence and Immunolectron microscopy),
5: MTT-Assay,
6: Gelelectrophoresis, Western- and Immunoblotting,
7: Immunoprecipitation.

