AG Prof. Waschke
Desmosome diseases
For the last two decades, we were investigating the cross-talk between cadherin-mediated cell adhesion and intracellular signaling in different cell types and tissues.
Now, we focus on the role of desmosomes in the pathophysiology of disease and how regulation of desmosome integrity and function can be targeted to treat disorders caused by dysfunction of desmosomes.
Desmosome diseases are predominantly caused by desmosome dysfunction which affects the adhesive and signaling functions of desmosomal contacts. Since desmosomes are most abundant in tissues subjected to high mechanical strain such as multilayered epithelia of the epidermis and mucous membranes and also in the myocardium, desmosome diseases disturb epithelial barrier integrity and cause arrhythmia and heart failure.
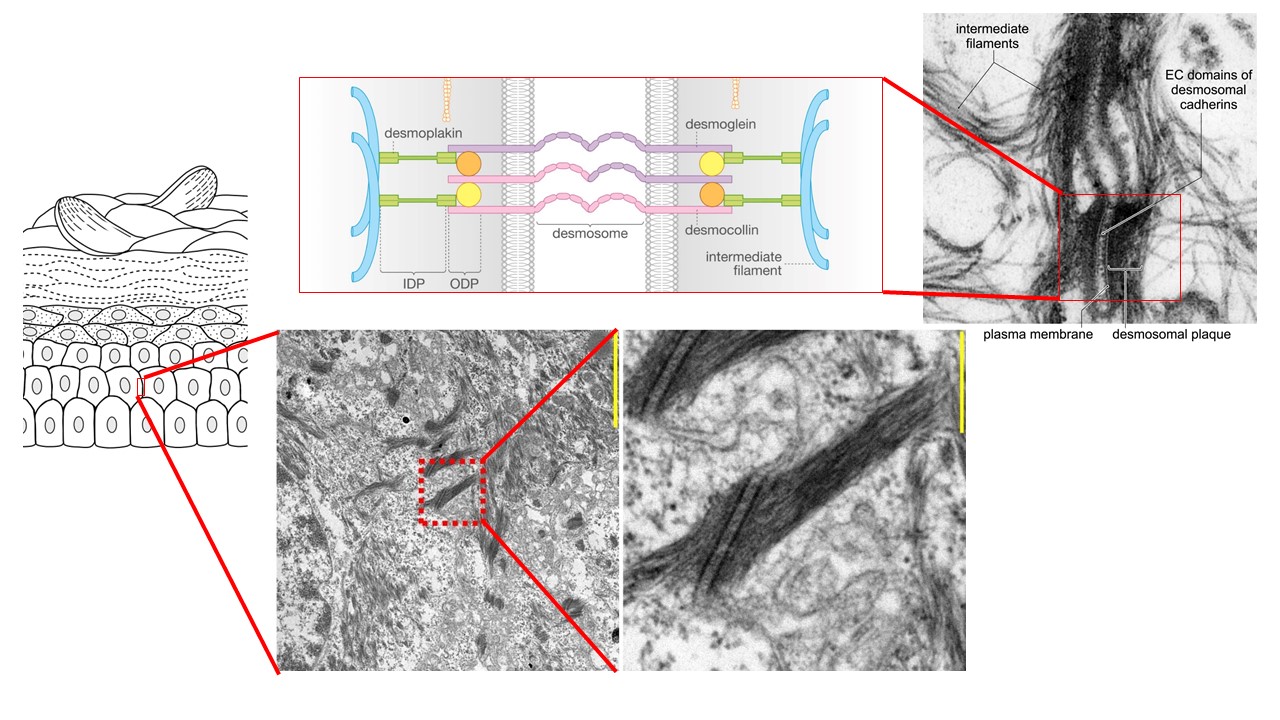
The prototype of an epithelial desmosome disease is the autoimmune blistering disease pemphigus. Here, autoantibodies mainly against the desmosomal cadherins desmoglein (Dsg) 1 and Dsg 3 interfere with desmosome turnover leading to loss of intercellular adhesion which ultimately causes the formation of epidermal and mucosal blisters and erosions.
Epidermal cell cohesion and pemphigus
Another desmosome disease is Arrhythmogenic cardiomyopathy (AC), which is believed to be caused primarily by genetic alterations of desmosomal components including Dsg2 but also plaque proteins such as plakophilin 2, desmoplakin and plakoglobin. In addition to genetic variants in these proteins, the pathogenic role of which in many cases is unclear, catalytic autoantibodies are detected in AC patients which cleave the adhesive proteins Dsg2 and N-cadherin.
Regulation of cardiomyocyte cohesion and arrhythmogenic cardiomyopathy
The structural backbone of desmosome consisting of desmosomal cadherins, plaque proteins and intermediate filaments is conserved in all tissues. Moreover, at least some mechanisms regulating desmosome adhesion and turnover are conserved between different cell types. Therefore, we propose that certain paradigms to stabilize desmosome integrity and function can be used to treat desmosome diseases and even may be applied in different tissues (Link: Spindler and Waschke, ADDM, JCS 2023, J Cell Sci. 2023 Jan 1;136(1):jcs260832. doi: 10.1242/jcs.260832. Epub 2023 Jan 3. PMID: 36594662).
We aim to further characterize the mechanisms involved in the regulation of desmosomes to better understand the requirements for proper desmosome function. From this, the ultimate goal is to gain insight in the pathophysiology of desmosome-related disorders and to establish new strategies for treatment of these diseases.
If this is interesting for you, connect to us to join our group!
In the past, one of our main research topics was regulation of endothelial barrier function, which is compromised during acute inflammation and sepsis. In this project, we established that endothelial barrier function and VE-cadherin binding are regulated mainly by a pathway including the Rho family GTPase Rac1, which is controled by cAMP signaling and involves regulation of actin cytoskeleton dynamics. Several basic principles in this scenario were later found to also apply to desmosome regulation.

