Regulation of cardiomyocyte cohesion and arrhythmogenic cardiomyopathy
Members:
Sunil Yeruva
Konstanze Stangner (research focus: iPSC cardiomyocytes)
Sina Moztarzadeh (research focus: animal models)
Kilian Skowranek (Technician)
Soumyata Pathak (PhD Student)
Clarissa Elsner (MD student)
Stephanie Wider (PhD student)
Project description:
The myocardium predominantly consists of cardiomyocytes, which require strong coupling to maintain heart function. Adjacent cardiomyocytes are coupled via intercalated disc (ICD) consisting of desmosomes, adherens junctions (AJ), and gap junctions, which together allow mechanical stability and electrical conduction of the heart muscle (Figure 1). The ICD of the cardiomyocyte consists of plicate and interplicate regions. The plicate region consists of the desmosome, as an individual mechanical component, and area composita or fascia adherens where both the desmosomes and adherens junction proteins are intermingled. Desmosomes are formed by the homo or heterophilic interactions of desmoglein 2 (DSG2) and desmocollin 2 (DSC2), where AJs are formed by the N-cadherin (N-cad). Desmosomes are anchored to the intermediate filament protein, desmin via the armadillo proteins plakoglobin (PG) also known as γ-catenin and plakophillin (PKP) 2 which further interacts with the plaque protein desmoplakin, which then interacts with desmin. AJs are very well known to be anchored to the actin cytoskeleton via the alpha-catenin (α), beta-catenin (β) and gamma-catenin (also known as PG). But in the area composita, it could also be likely that desmosome and AJs are intermingled in such a way that desmosomes are anchored to the actin cytoskeleton and AJs to the desmin. The interplicate region has two regions, perinexus and nexus, respectively. In the perinexus region, sodium channel complexes (Nav 1.5) concentrated at the edge of GJs, which are found in the nexus region, are involved in ephaptic conduction in the heart. The sodium channel β1 subunit with its extracellular adhesion domain, plays a critical role in this mechanism. Another important ICD structure that plays a major role in cardiomyocyte adhesion is the β-adrenergic receptor (Figure 1).
Arrhythmogenic cardiomyopathy (AC) is a familial heart disease also known as 'the disease of desmosome' since more than 50% of AC patients carry mutations that affect the desmosomal components of the ICD and impair desmosome turnover, which eventually weakens cellular cohesion, leading to arrhythmias, cardiac injury, fibrosis and adipogenesis. Further, only symptomatic treatments, such as β-blockers are employed in most of the AC patients. Therefore, we are interested in investigating how cardiomyocyte cohesion is regulated under pathophysiological conditions, with a major focus on finding new therapeutic targets for AC.
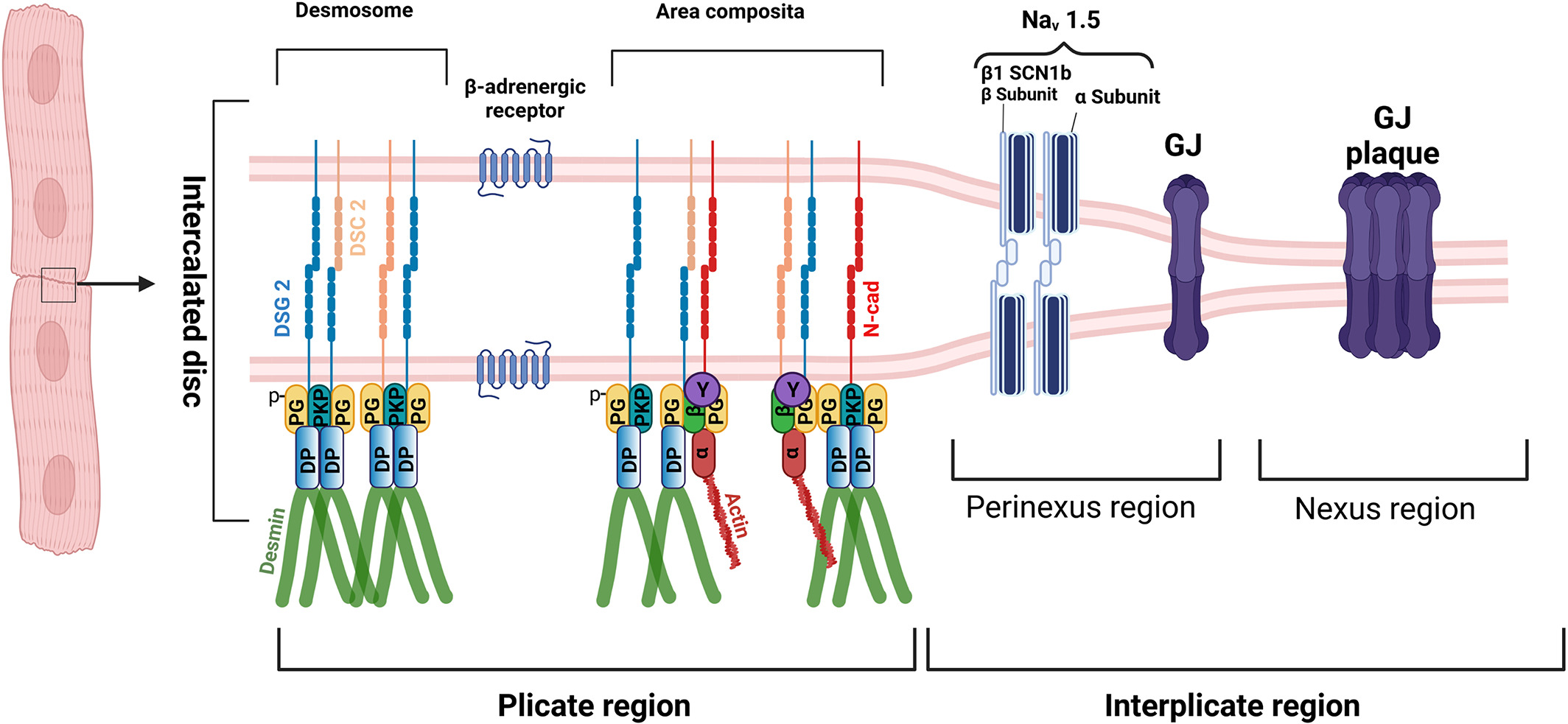
Figure 1: Structure of the intercalated disc (from Yeruva and Waschke, J. Anatomy 2023)
Our major findings, as illustrated in figure 2, are that adrenergic signaling (F/R and Iso), PKC activation (PMA), and p38MAPK inhibition (SB20) enhance basal cardiomyocyte cohesion, which we referred to as 'positive adhesiotropy' in ERK1/2-dependent manner. Positive adhesiotropy is possibly achieved through alterations in the interactions of the desmosomal proteins PG, PKP2, and DP that eventually lead to increased DSG2 translocation to the cell borders and finally to positive adhesiotropy (Shoyket et al., JCI Insight 2020, Yeruva and Waschke, J. Anatomy 2023). On the other hand, adrenergic signaling also acts via PKA-dependent PG phosphorylation at S665 (Schinner et al., Circ res 2017), leading to an ultrastructural strengthening of intercalated discs and thereby increasing basal cardiomyocyte cohesion (Yeruva et al., Front Physiol 2020). In addition to the above mechanisms, we have also studied the role of the ionotropic agent digitoxin on cardiomyocyte adhesion and observed that digitoxin induces the recruitment of desmosomal proteins with the strengthening of the mechanical coupling of the ICD via ERK1/2 activation (Schinner et al., Basic Res. Cardiol. 2020). approach to treat AC (Schinner et al., JCI Insight 2020).
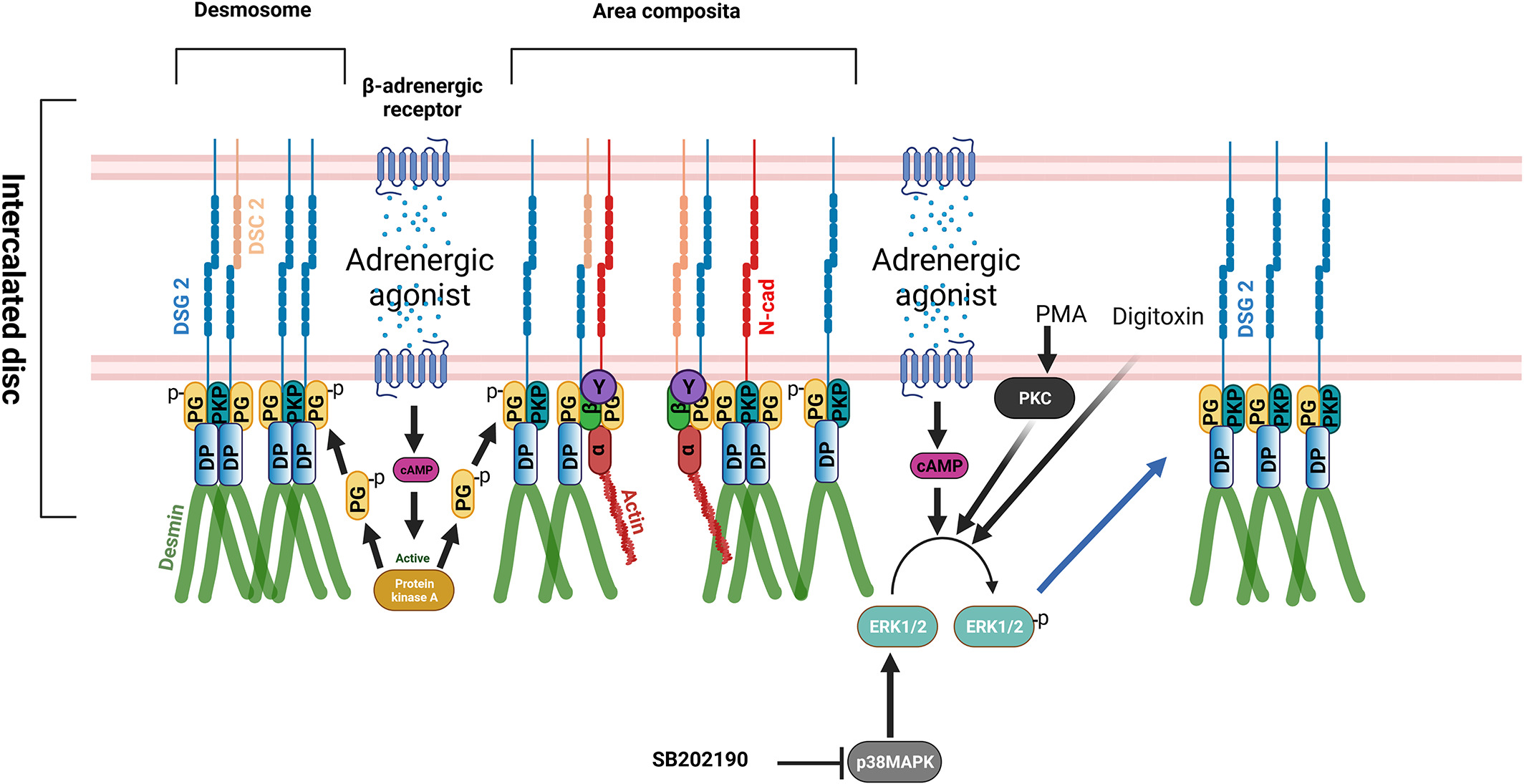
Figure 2: Signaling mechanisms regulating cardiomyocyte cohesion (from Yeruva and Waschke, J. Anatomy 2023)
Since we found several signaling pathways that enhance cardiomyocyte cohesion, we further investigated the signaling pathways impairing cardiomyocyte cohesion. As cardiac stimulation by the parasympathetic nervous system (PNS) is associated with reduced chronotropy and inotropy and is known to act antagonistically to the sympathetic nervous system, we hypothesized that cardiomyocyte cohesion by the PNS could be regulated oppositely. Therefore, we investigated the role of cholinergic signaling in cardiomyocyte cohesion. By utilizing carbachol as a cholinergic agonist mimicking the PNS, we established for the first time that cholinergic signaling activation in both HL-1 cells and murine ventricular cardiac slices from wild-type and an AC murine model (Jup-/- mice), antagonizes the 'positive adhesiotropy' of adrenergic signaling on cardiomyocyte cohesion and thus causes 'negative adhesiotropy,' which is independent of PG phosphorylation [figure 3] (Yeruva et al., Acta Physiologica 2022, Vielmuth et al., Acta Physiologica 2023 ).
The cardiac desmosome is classically thought to function as a cell–cell adhesive structure; however, emerging evidence points to non-canonical roles for the cardiac desmosome in regulating electrical channels and function, independent of its structural roles. Indeed, we found a functional interplay between desmosomes, GJ protein, and β1-adrenergic receptors in HL-1 cardiomyocytes [figure 3] (Schinner et al., Acta Physiolgica 2019).
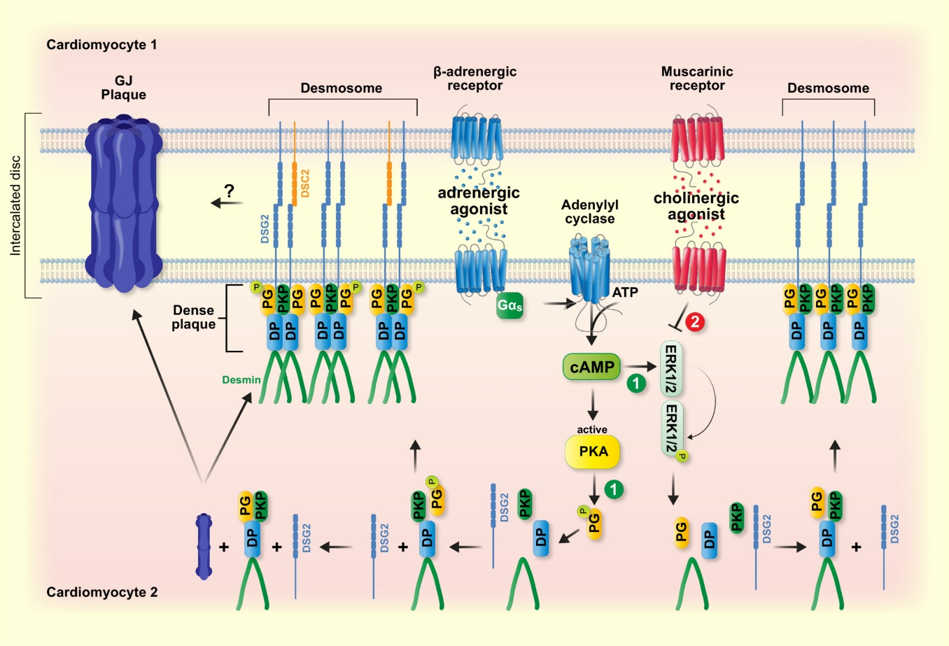
Figure 3: cAMP-mediated stabilization of desmosomal adhesion at intercalated discs. Adrenergic and cholinergic signaling have antagonistic effects on desmosomal adhesion which in part are mediated by opposite regulation of signaling molecules such as ERK. In addition, cAMP triggers PKA-mediated Pg phosphorylation at S665, which drives desmosome assembly and enhances GJ function. (from Vielmuth et al., Acta Physiologica 2023)
Therefore, improving cardiomyocyte cohesion may effectively rescue impaired electrical conduction. We already proved in the pemphigus field that a peptide-based stabilization of intercellular cohesion was protective, where autoantibodies against DSG3 impair keratinocyte cohesion with induction of skin blistering. Thus, an analog approach could serve as a novel therapeutic target for treating arrhythmias in AC. To explore this avenue, we studied whether a desmoglein 2-linking peptide (DSG2-LP) could rescue the arrhythmias. We demonstrated for the first time that stabilization of DSG2-mediated binding by a specific linking peptide can rescue both cardiomyocyte cohesion and GJ-mediated signal transduction caused by disruption of intercellular adhesion. Because arrhythmia in perfused hearts derived from an AC mouse model was successfully reduced by DSG2-LP treatment, these results indicate that stabilization of Dsg2 binding can be an effective approach to treat AC (Schinner et al., JCI Insight 2020).
Previous studies from other research groups demonstrated the activation of epidermal growth factor receptor (EGFR) by cholinergic signaling in epithelial tissues. Furthermore, selective muscarinic receptor (MR) 1 agonist AF267B treatment reduced amyloid-β peptide through enhanced steady-state levels of ADAM17, whereas in cells overexpressing MR 3 receptor, inhibition of ADAM17 inhibited MR 3 receptor coupled to the release of the amyloid precursor protein ectodomain. In line with these observations, utilizing two different murine AC models (Jup-/- and Pkp2-/-) and AC patients' cardiac tissue lysates, we found an increase in EGFR protein (Shoykhet et al., JCI Insight 2023), inhibition of which enhanced cardiomyocyte cohesion via ROCK pathway (figure 4). Further, we have demonstrated that the inhibition of ADAM17, an upstream EGFR pathway effector molecule, enhanced cardiomyocyte cohesion by up-regulating DP and DSG2 at the cell borders (Shoykhet et al., Front Cell Dev Biol. 2023).
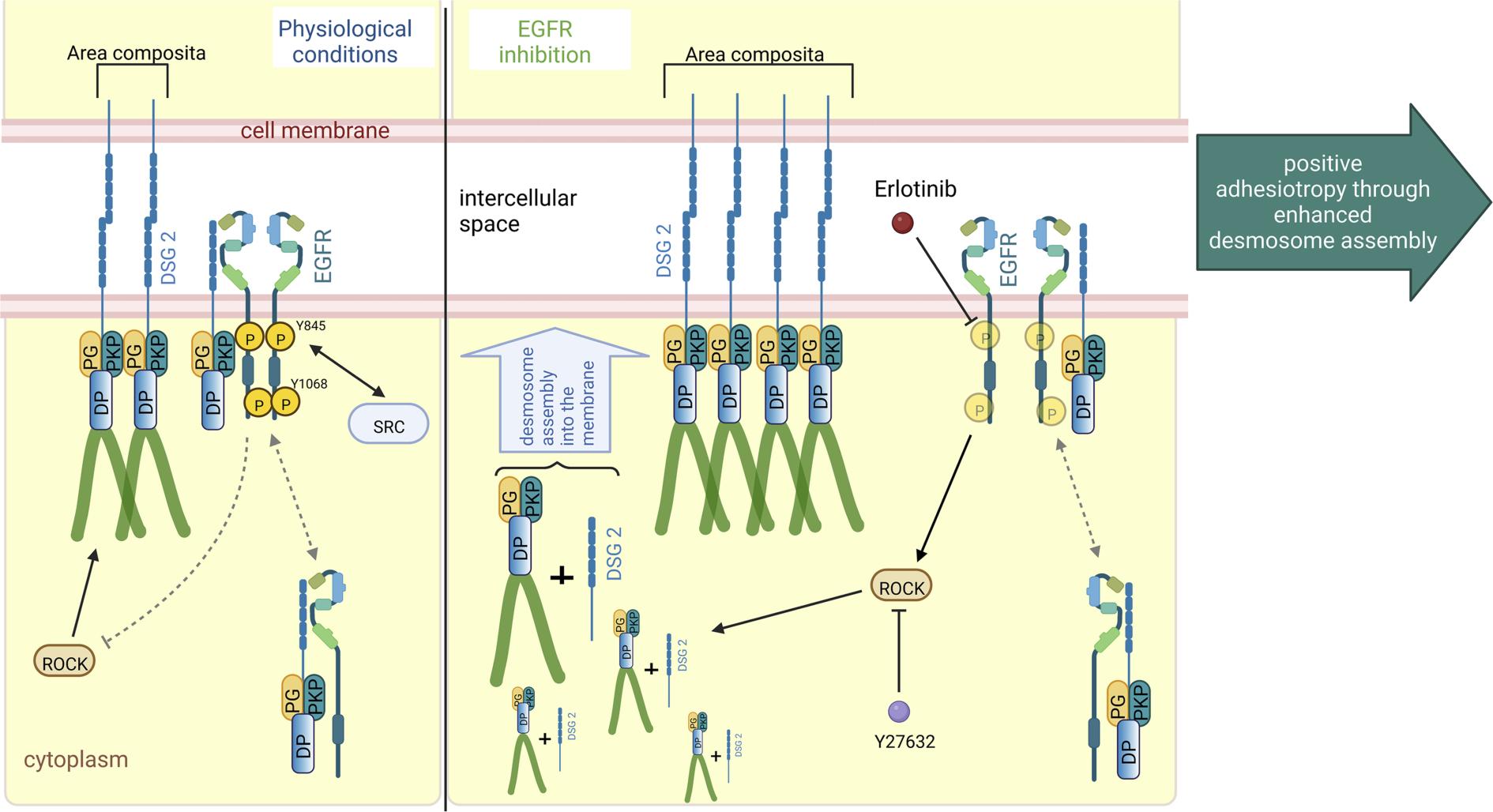
Figure 4: Schematic overview of EGFR inhibition–mediated positive adhesiotropy
EGFR exists in complex with DSG2, along with DP, PG, and PKP2, either at the cell borders or in the cytoplasm. Under physiological conditions, ROCK is necessary for basal cardiomyocyte cohesion. EGFR inhibition by erlotinib leads to ROCK activation, which results in positive adhesiotropy via enhanced desmosome assembly and area composita length. Inhibition of ROCK completely abrogates erlotinib-mediated positive adhesiotropy. (from Shoykhet et al., JCI Insight 2023)
As said earlier, AC is a familial disease, in which more than 50% of patients carry mutations in genes coding for desmosomal proteins of the ICD. However, no genetic cause can be determined for a substantial number of patients, and the course of the disease can differ significantly in families with the same underlying mutation; thus, the genetic predisposition alone cannot explain the pathogenesis of AC. In this context, it is highly relevant that autoantibodies targeting DSG2 have been described in AC patients, irrespective of the AC causing gene mutations. In this regard, we have recently demonstrated for the first time that IgGs in AC patients exhibit catalytic activity against DSG2 and N-CAD effectively reducing interaction of these adhesion molecules. Furthermore, most AC-IgGs that caused loss of cardiomyocyte cohesion induce activation of p38MAPK, whereas inhibition of the p38MAPK restored the loss of cardiomyocyte cohesion caused by the former [figure 5] (Yeruva et al., Cellular and Molecular Life Sciences 2023).
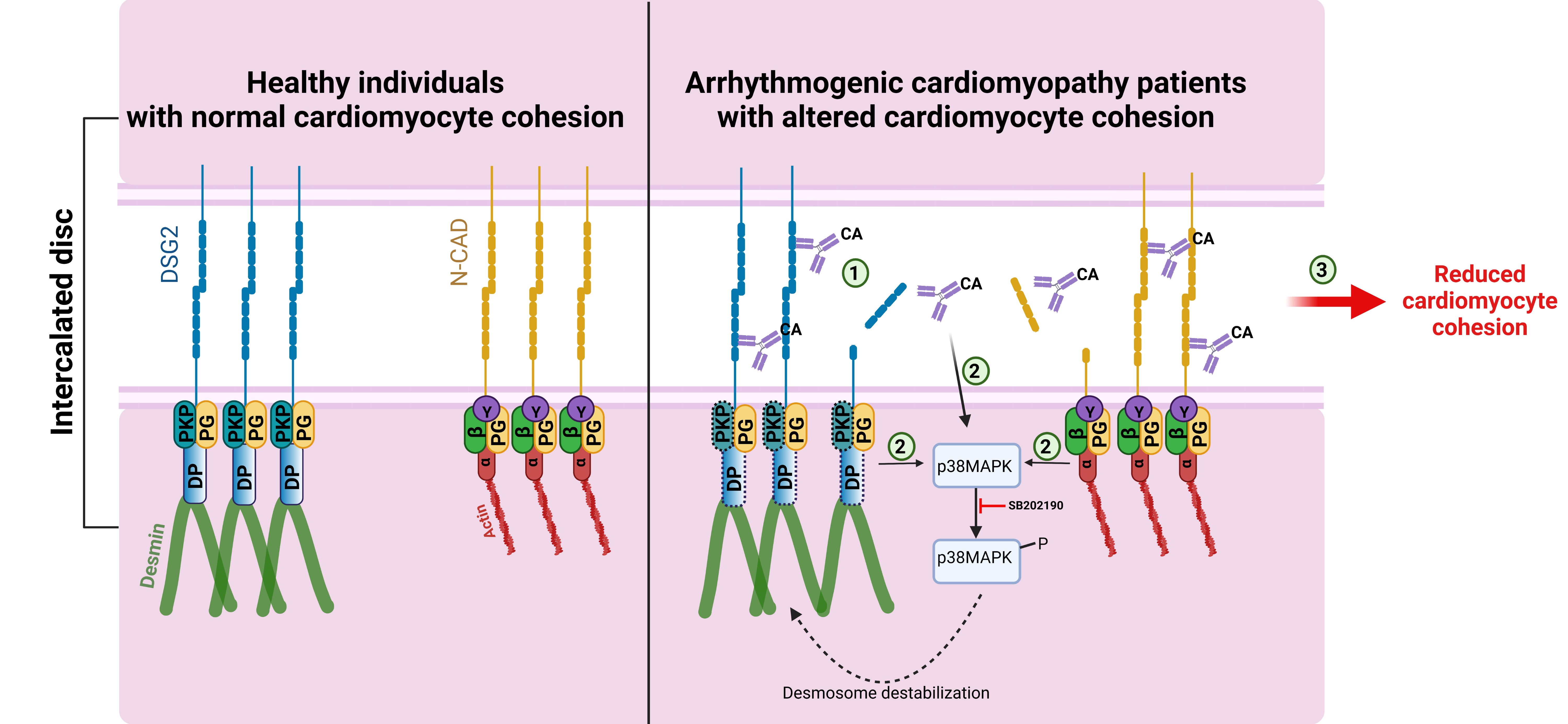
Figure 5: Catalytic antibodies mediated cardiomyocyte cohesion in AC patients.
In normal healthy individuals, the cadherin family proteins, desmoglein 2 (DSG2) and N-cadherin (N-CAD) proteins are properly localized to the ICD via the plaque proteins DSP and PKP2 and armadillo proteins PG, α-catenin (α), β-catenin (β) and YAP (Y), respectively. In AC patients, having mutations in either DSP or PKP2 genes (the corresponding proteins are represented with dashed boxes), presence of catalytic antibodies (CA) that can cleave DSG2 or N-CAD could destabilize the cadherin-mediated adhesion (1), which could lead to the activation of p38MAPK (2), which further can lead to desmosome destabilization. Cleavage of cadherin proteins combined with p38MAPK activation could eventually result in reduced cardiomyocyte cohesion (3) and thereby a reduction in the mechanical strengths of the cardiomyocyte. ( from Yeruva et al., Cellular and Molecular Life Sciences 2023)
Future Perspectives:
We have now found that targeting the cAMP or EGFR signaling pathways is beneficial in rectifying the impaired cardiomyocyte cohesion under AC pathological conditions. We are now focusing on translating these observations into humans. For these reasons, we are using human iPSC-derived cardiomyocytes from healthy donors and AC patients and will evaluate our previous observations, as mentioned above.
For Medical students: Medical students of LMU or TU interested in our projects are always welcome to work with us for their doctoral thesis. You can follow the below link for further information.
https://www.med.lmu.de/promotion/doktorarbeiten/index.html
Publications:
Williams T, Groß R, Arias-Loza AP, Nordbeck P, Noerpel M, Cirnu A, Kimmel L, Ashour D, Ramos G, Waschke J, Higuchi T, Gerull B (2025)
Illuminating Cardiac Remodeling: Insights From [18F]-Fluorodeoxyglucose Positron Emission Tomography Imaging in Plakoglobin-Associated Arrhythmogenic Cardiomyopathy.
J Am Heart Assoc.;14(5):e038331. doi: 10.1161/JAHA.124.038331.
Yeruva S, Stangner K, Jungwirth A, Hiermaier M, Shoykhet M, Kugelmann D, Hertl M, Egami S, Ishii N, Koga H, Hashimoto T, Weis M, Beckmann BM, Biller R, Schüttler D, Kääb S, Waschke J.
Catalytic antibodies in arrhythmogenic cardiomyopathy patients cleave desmoglein 2 and N-cadherin and impair cardiomyocyte cohesion.
Cell Mol Life Sci. 2023 Jul 14;80(8):203. doi: 10.1007/s00018-023-04853-1. PMID: 37450050
Shoykhet M, Dervishi O, Menauer P, Hiermaier M, Moztarzadeh S, Osterloh C, Ludwig RJ, Williams T, Gerull B, Kääb S, Clauss S, Schüttler D, Waschke J, Yeruva S (2023)
EGFR inhibition leads to enhanced desmosome assembly and cardiomyocyte cohesion via ROCK activation.
JCI Insight. 2023 Mar 22;8(6):e163763. doi: 10.1172/jci.insight.163763. PMID: 36795511
Shoykhet M, Waschke J, Yeruva S (2023)
Cardiomyocyte cohesion is increased after ADAM17 inhibition.
Front Cell Dev Biol. 2023 Jan 17;11:1021595. doi: 10.3389/fcell.2023.1021595. eCollection 2023. PMID: 36733457
Yeruva S, Waschke J (2023)
Structure and regulation of desmosomes in intercalated discs: Lessons from epithelia.
J Anat. 2023 Jan;242(1):81-90. doi: 10.1111/joa.13634. Epub 2022 Feb 6. PMID: 35128661
Yeruva S, Körber L, Hiermaier M, Egu DT, Kempf E, Waschke J (2022)
Cholinergic signaling impairs cardiomyocyte cohesion.
Acta Physiol (Oxf). 2022 Nov;236(3):e13881. doi: 10.1111/apha.13881. Epub 2022 Sep 16. PMID: 36039679
Shoykhet M, Trenz S, Kempf E, Williams T, Gerull B, Schinner C, Yeruva S, Waschke J
Cardiomyocyte adhesion and hyperadhesion differentially require ERK1/2 and plakoglobin.
JCI Insight. 2020 Sep 17;5(18):e140066. doi: 10.1172/jci.insight.140066. PMID: 32841221
Schinner C, Olivares-Florez S, Schlipp A, Trenz S, Feinendegen M, Flaswinkel H, Kempf E, Egu DT, Yeruva S, Waschke J (2020)
The inotropic agent digitoxin strengthens desmosomal adhesion in cardiac myocytes in an ERK1/2-dependent manner.
Basic Res Cardiol. 2020 Jun 17;115(4):46. doi: 10.1007/s00395-020-0805-3. PMID: 32556797
Yeruva S, Kempf E, Egu DT, Flaswinkel H, Kugelmann D, Waschke J (2020)
Adrenergic Signaling-Induced Ultrastructural Strengthening of Intercalated Discs via Plakoglobin Is Crucial for Positive Adhesiotropy in Murine Cardiomyocytes.
Front Physiol. 2020 May 21;11:430. doi: 10.3389/fphys.2020.00430. eCollection 2020. PMID: 32508670.
Schinner C, Erber BM, Yeruva S, Schlipp A, Rötzer V, Kempf E, Kant S, Leube RE, Mueller TD, Waschke J (2020)
Stabilization of desmoglein-2 binding rescues arrhythmia in arrhythmogenic cardiomyopathy
JCI Insight. 2020 May 7;5(9):e130141. doi: 10.1172/jci.insight.130141. PMID: 32376797.
Schinner C, Erber BM, Yeruva S, Waschke J (2018)
Regulation of cardiac myocyte cohesion and gap junctions via desmosomal adhesion.
Acta Physiol (Oxf). Dec 23:e13242. Epub ahead of print. PMID: 30582290.
Schinner C, Vielmuth F, Rötzer V, Hiermaier M, Radeva MY, Co TK, Hartlieb E, Schmidt A, Imhof A, Messoudi A, Horn A, Schlipp A, Spindler V, Waschke J (2017)
Adrenergic Signaling Strengthens Cardiac Myocyte Cohesion.
Circ Res.120(8):1305-1317. PMID: 28289018.
Schlipp A, Schinner C, Spindler V, Vielmuth F, Gehmlich K, Syrris P, Mckenna WJ, Dendorfer A, Hartlieb E, Waschke J (2014)
Desmoglein-2 interaction is crucial for cardiomyocyte cohesion and function.
Cardiovasc Res, 104(2):245-57. PMID: 25213555

