Epidermal cell cohesion and pemphigus
Members:
Anna Sigmund (research focus: Mechanisms stabilizing desmosome adhesion)
Thomas Schmitt (group leader, research focus: Dsg-mediated signaling)
Michael Fuchs (research focus: Biophysical characterization of keratinocyte adhesion)
Desalegn Egu (research focus: Ultrastructural Analysis of desmosomes by electron microscopy)
Mariya Radeva (research focus: mouse models)
Alexander Garcia Ponce (research focus: epidermal barrier regulation)
Martina Hitzenbichler (technician)
Sabine Mühlsimer (technician)
Marlene Katzmann (technician)
Bodenstaff Frank (technician)
Paulina Rion (MD student)
The epidermis represents the outermost barrier sealing our body against the environment. The integrity of these stratifying cell layers depends on desmosomes, specific intercellular junction complexes designed to tether adjacent keratinocytes together. This enables the epidermis to withstand the high mechanical forces this tissue constantly is exposed to. The core of desmosomes is composed of the cadherin-type transmembrane adhesion molecules desmogleins and desmocollins. These molecules are intracellularly linked to the intermediate filament network by the adapter proteins plakoglobin, plakophilins and desmoplakin (Fig. 1). .
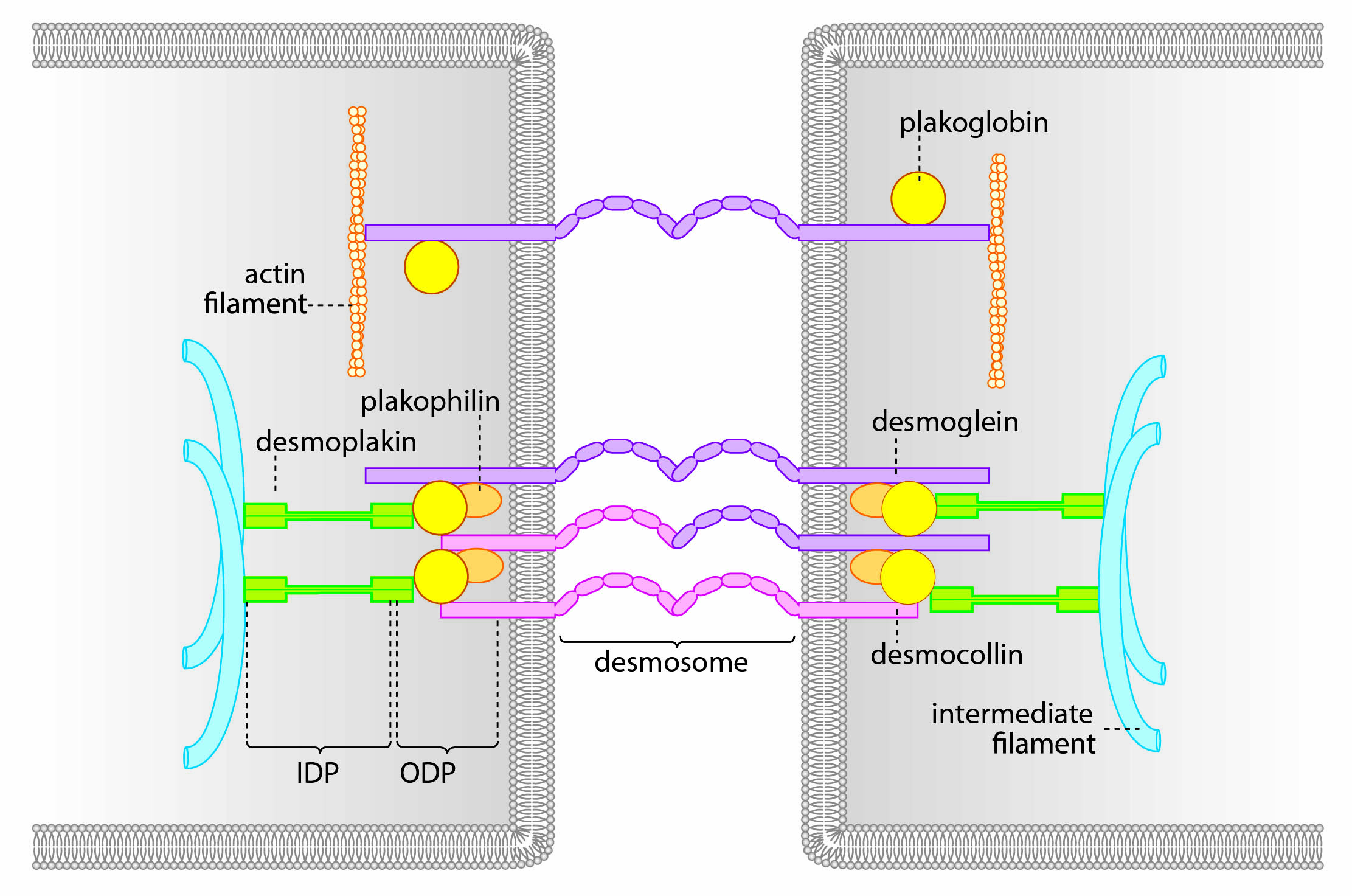
Fig. 1 Schematic of a desmosome
Specifically desmogleins are known to exist “free” on the membrane without attachment in the desmosome. These molecules are connected to a variety of signaling molecules such as Rho-GTPases, Src and p38MAPK. By regulating the activity of these molecules, extradesmosomal desmogleins serve as signaling hubs to modulate cellular behavior (Fig. 2).
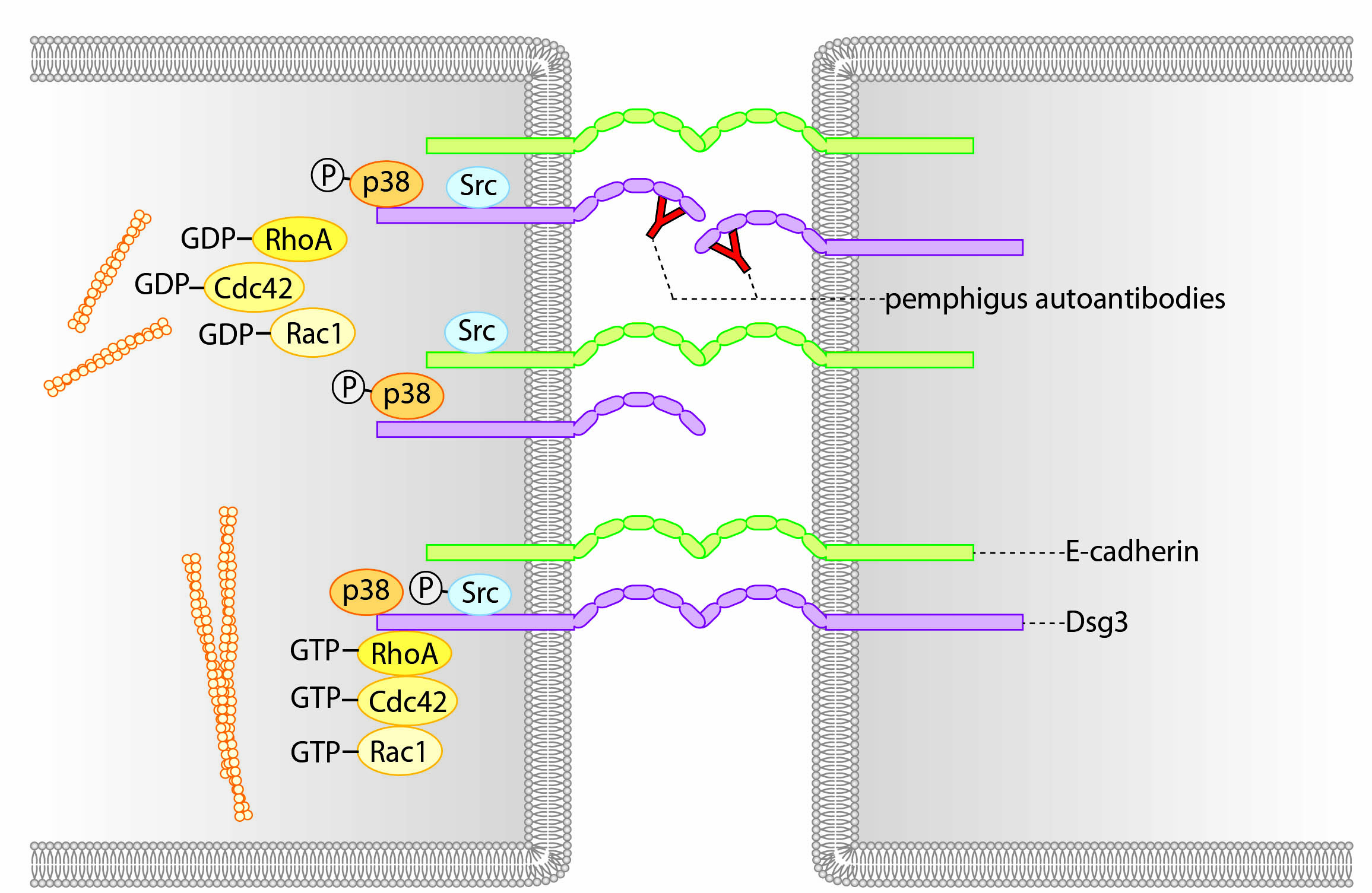
Fig. 2 Extradesmosomal desmoglein molecules serve as signaling hubs
The importance of desmosomal adhesion becomes evident by the autoimmune disease pemphigus. The main clinical variant is pemphigus vulgaris. Patients develop autoantibodies against the desmoglein isoforms 3 and 1 (Dsg3 and Dsg1) resulting in loss of desmosomal function. This loss of cell cohesion leads to cleft formation within the epidermis and the mucosa of the oral cavity. Macroscopically, blisters are visible which rupture rapidly (Fig.3). Without immunosuppression, the disease is lethal.
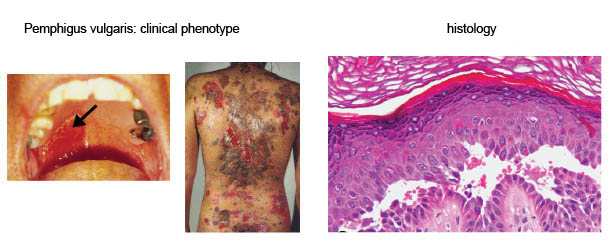
Fig. 3: Pemphigus
Binding of pemphigus autoantibodies leads to altered intracellular signaling, at least in part following initial loss of Dsg3 interaction (Fig. 4). This induces (i) loss of Dsg3 and other desmosomal molecules from the cell surface and (ii) uncoupling of the intermediate filaments from the desmosome. These two effects are thought to represent the main mechanisms of loss of cell-cell adhesion and ultimately blister formation. Currently, we focus on signaling patterns induced by autoantibodies from patients with different pemphigus phenotypes.
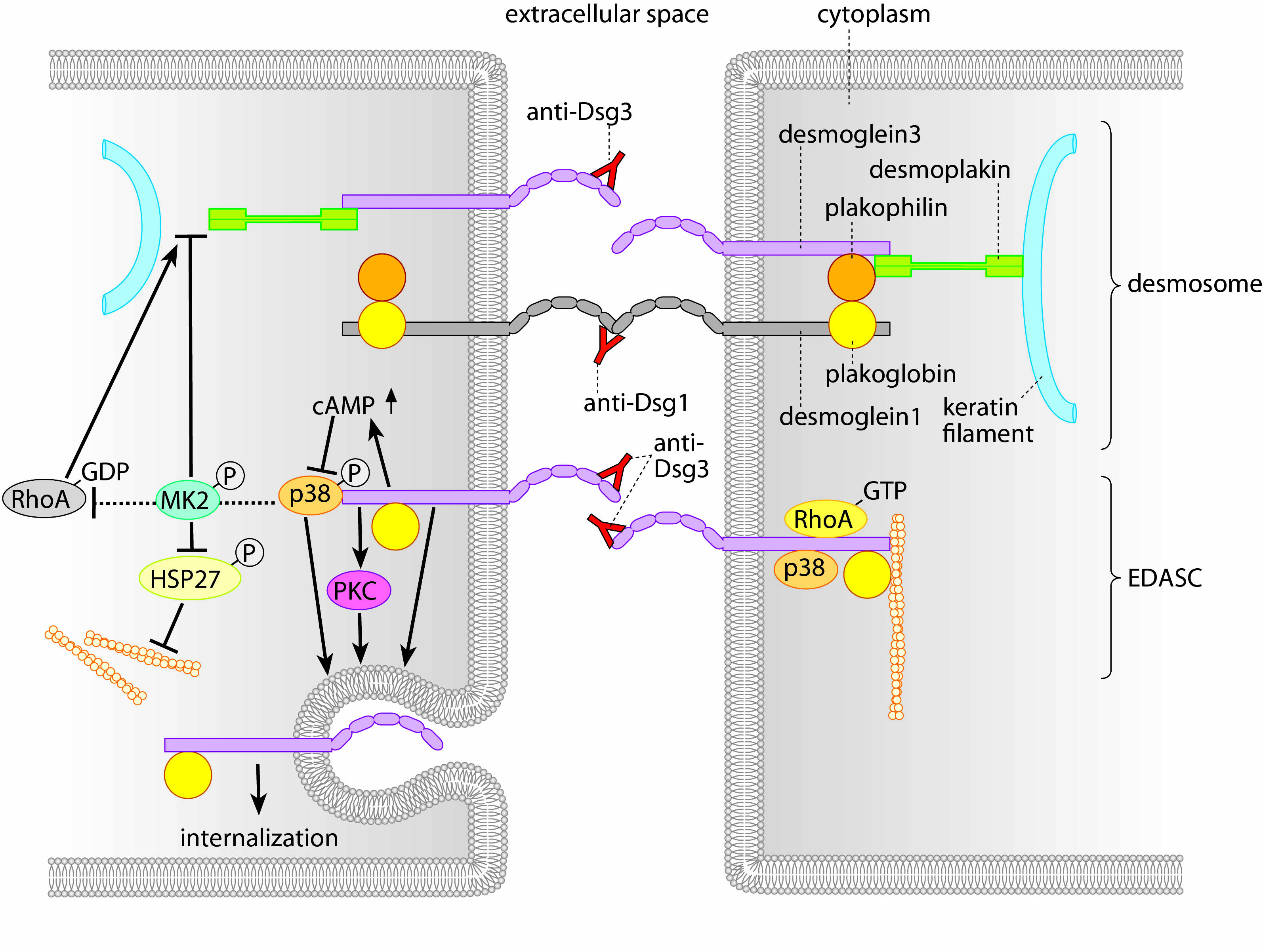
Fig. 4: Mechanisms of blister formation in pemphigus
Within the project we investigate the regulation of desmosomal adhesion. Specifically, we address how desmosomes are assembled and disassembled and which mechanisms and signals are involved in these processes. Furthermore, we aim to better understand the mechanisms that lead to loss of cell cohesion and blister formation in pemphigus to identify novel targets as potential treatment options for this life-threatening disease.
We combine state-of-the-art biochemical and molecular biology techniques with morphological assessment of desmosomes and their turnover (using live cell imaging, confocal microscopy, transmission and scanning electron microscopy). To correlate this with the adhesive function of desmosomes, we apply adhesion assays and biophysical techniques such as atomic force microscopy and optical tweezers (see here).
Recent publications (selection since 2013):
Fuchs M, Möchel M, Radeva MY, Schmitt T, Yazdi AS, Hashimoto T, Waschke J (2025)
In desmosomes direct inhibition precedes p38MAPK-mediated uncoupling to reduce Dsg3 adhesion by pemphigus autoantibodies.
Br J Dermatol.:ljaf142. doi: 10.1093/bjd/ljaf142. PMID: 40264374
Hiermaier M, Egu DT, Sigmund AM, Hertl M, Ghoreschi K, Schmidt E Hashimoto T, Waschke J (2025)
The multi-kinase inhibitor midostaurin mitigates loss of intercellular adhesion and skin blistering in pemphigus vulgaris.
J. Invest. Dermatol, accepted
Sigmund AM, Bayerbach FC, Kugelmann D, Butz E, Moztarzadeh S, Schikora ME, Horn AK, Radeva MY, Engelmayer S, Egu DT, Goebeler M, Schmidt E, Waschke J, Vielmuth F (2025)
Epac1 contributes to apremilast-mediated rescue of pemphigus autoantibody-induced loss of keratinocyte adhesion.
JCI Insight.:e187481. doi: 10.1172/jci.insight.187481. PMID: 40299565 Free article.
Schmitt T, Huber J, Pircher J, Schmidt E, Waschke J (2025)
The impact of signaling pathways on the desmosome ultrastructure in pemphigus.
Front Immunol.;15:1497241. doi: 10.3389/fimmu.2024.1497241. PMID: 39882246 Free PMC article.
Waschke J, Amagai M, Becker C, Delmar M, Duru F, Garrod DR, Gerull B, Green KJ, Hertl M, Kowalczyk AP, Niessen CM, Nusrat A, Schinner C, Schlegel N, Sivasankar S, Vielmuth F, Spindler V (2025)
Meeting report - Alpine desmosome disease meeting 2024: advances and emerging topics in desmosomes and related diseases.
J Cell Sci. 15;138(2): JCS263796. doi: 10.1242/jcs.263796. PMID: 39838950
Waschke J (2025)
Pathogenic pemphigus autoantibodies against desmosomal cadherins target the intracellular domain of desmocollin 3: implications for pemphigus pathogenesis, diagnosis and treatment!
Br J Dermatol.:ljaf034. doi: 10.1093/bjd/ljaf034.
Emtenani S, Baines JF, Bieber K, Gaffal E, Goletz S, Hernández G, Hirose M, Hoffmann M, Joly P, Kirchner H, Köhl J, Murthy S, Patzelt S, Petersen F, Pigors M, Riemekasten G, Schmidt-Jiménez LF, Sezin T, Spielmann M, Thaçi D, van Beek N, Waschke J, Ludwig RJ, Schmidt E (2025)
Meeting Report on "10th Anniversary Symposium on Inflammatory Skin Disease".
JID Innov.;5(3):100344. doi: 10.1016/j.xjidi.2024.100344.
Steinert L, Fuchs M, Sigmund AM, Didona D, Hudemann C, Möbs C, Hertl M, Hashimoto T, Waschke J, Vielmuth F (2024)
Desmosomal hyper-adhesion affects direct inhibition of desmoglein interactions in pemphigus.
J Invest Dermatol. 144(12):2682-2694.e10. doi: 10.1016/j.jid.2024.03.042.
Egu DT, Schmitt T, Ernst N, Ludwig RJ, Fuchs M, Hiermaier M, Moztarzadeh S, Morỏn CS, Schmidt E, Beyersdorfer V, Spindler V, Steinert LS, Vielmuth F, Sigmund AM, Waschke J (2024)
EGFR Inhibition by Erlotinib Rescues Desmosome Ultrastructure and Keratin Anchorage and Protects Against Pemphigus Vulgaris IgG-Induced Acantholysis in Human Epidermis.
J Invest Dermatol. 44(11):2440-2452. doi: 10.1016/j.jid.2024.03.040 PMID: 38642796 Free article.
Sigmund AM, Winkler M, Engelmayer S, Kugelmann D, Egu DT, Steinert LS, Fuchs M, Hiermaier M, Radeva MY, Bayerbach FC, Butz E, Kotschi S, Hudemann C, Hertl M, Yeruva S, Schmidt E, Yazdi AS, Ghoreschi K, Vielmuth F, Waschke J (2023)
Apremilast prevents blistering in human epidermis and stabilizes keratinocyte adhesion in pemphigus.
Nat Comm, 14(1):665. doi: 10.1038/s41467-023-36464-6. PMID: 36750581 Free PMC article. No abstract available.
Spindler V, Gerull B, Green KJ, Kowalczyk AP, Leube R, Marian AJ, Milting H, Müller EJ, Niessen C, Payne AS, Schlegel N, Schmidt E, Strnad P, Tikkanen R, Vielmuth F, Waschke J (2023)
Meeting report - Desmosome dysfunction and disease: Alpine desmosome disease meeting.
J Cell Sci. 136(1):jcs260832. doi: 10.1242/jcs.260832. PMID: 36594662
Fuchs M, Radeva MY, Spindler V, Vielmuth F, Kugelmann D, Waschke J (2023)
Cytoskeletal anchorage of different Dsg3 pools revealed by combination of hybrid STED/SMFS- AFM.
Cell Mol Life Sci. 80(1):25. doi: 10.1007/s00018-022-04681-9. PMID: 36602635 Free PMC article.
Schmitt T, Hudemann C, Moztarzadeh S, Hertl M, Tikkanen R, Waschke J (2023)
Dsg3 epitope-specific signalling in pemphigus.
Front Immunol., 4:1163066. doi: 10.3389 PMID: 37143675 Free PMC article.
Vielmuth F, Radeva MY, Yeruva S, Sigmund AM, Waschke J (2023)
cAMP: A master regulator of cadherin-mediated binding in endothelium, epithelium and myocardium.
Acta Physiol, 238(4):e14006. doi: 10.1111/apha.14006 PMID: 37243909 Review.
Hudemann C, Exner Y, Pollmann R, Schneider K, Zakrzewicz A, Feldhoff S, Schmidt T, Spindler V, Rafei-Shamsabadi D, Völlner F, Waschke J, Tikkanen R, Hertl M, Eming R. (2023)
IgG against the Membrane-Proximal Portion of the Desmoglein 3 Ectodomain Induces Loss of Keratinocyte Adhesion, a Hallmark in Pemphigus Vulgaris.
J Invest Dermatol. 2023 Feb;143(2):254-263.e3. doi: 10.1016/j.jid.2022.07.030. Epub 2022 Sep 9.
PMID: 36089007 Free article.
Hudemann C, Maglie R, Llamazares-Prada M, Beckert B, Didona D, Tikkanen R, Schmitt T, Hashimoto T, Waschke J, Hertl M, Eming R. J (2022)
Human Desmocollin 3‒Specific IgG Antibodies Are Pathogenic in a Humanized HLA Class II Transgenic Mouse Model of Pemphigus.
Invest Dermatol. 142(3 Pt B):915-923.
Büchau F, Vielmuth F, Waschke J, Magin TM (2022) Bidirectional regulation of desmosome hyperadhesion by keratin isotypes and desmosomal components.
Cell Mol Life Sci. 79(5):223. PMID: 35380280 Free PMC article.
Fuchs M, Kugelmann D, Schlegel N, Vielmuth F, Waschke J (2022)
Desmoglein 2 can undergo Ca2+-dependent interactions with both desmosomal and classical cadherins including E-cadherin and N-cadherin
Biophys J. 2022 Apr 5;121(7):1322-1335. doi: 10.1016/j.bpj.2022.02.023. Epub 2022 Feb 17.PMID: 35183520 Free PMC article.
Egu DT, Schmitt T, Sigmund AM, Waschke J (2022)
Electron microscopy reveals that phospholipase C and Ca2+ signaling regulate keratin filament uncoupling from desmosomes in Pemphigus.
Ann Anat. 241:151904, 241:151904. doi: 10.1016/j.aanat.2022.151904.
Hiermaier M, Kugelmann D, Radeva MY, Didona D, Ghoreschi K, Farzan S, Hertl M, Waschke J (2022)
Pemphigus Foliaceus Autoantibodies Induce Redistribution Primarily of Extradesmosomal Desmoglein 1 in the Cell Membrane.
Front Immunol. 12;13:882116. PMID: 35634274 Free PMC article.
Schmitt T, Pircher J, Steinert L, Meier K, Ghoreschi K, Vielmuth F, Kugelmann D, Waschke J (2022)
Dsg1 and Dsg3 Composition of Desmosomes Across Human Epidermis and Alterations in Pemphigus Vulgaris Patient Skin.
Front Immunol. 25;13:884241. PMID: 35711465 Free PMC article.
Kugelmann D, Anders M, Sigmund AM, Egu DT, Eichkorn RA, Yazdi AS, Sárdy M, Hertl M, Didona D, Hashimoto T, Waschke J (2022)
Role of ADAM10 and ADAM17 in the Regulation of Keratinocyte Adhesion in Pemphigus Vulgaris.
Front Immunol. 13:884248. PMID: 35844545 Free PMC article.
Eichkorn RA, Schmidt MF, Walter E, Hertl M, Baron JM, Waschke J, Yazdi AS (2022)
Innate immune activation as cofactor in pemphigus disease manifestation.
Front Immunol. 13:898819.
Egu DT, Schmitt T, Sigmund AM, Waschke J (2022)
Electron microscopy reveals that phospholipase C and Ca2+ signaling regulate keratin filament uncoupling from desmosomes in Pemphigus.
Ann Anat. 2022 Apr;241:151904. doi: 10.1016/j.aanat.2022.151904. Epub 2022 Feb 4. PMID: 35131450
Hudemann C, Exner Y, Pollmann R, Schneider K, Zakrzewicz A, Feldhoff S, Schmidt T, Spindler V, Rafei-Shamsabadi D, Völlner F, Waschke J, Tikkanen R, Hertl M, Eming R. J (2022)
IgG against the Membrane-Proximal Portion of the Desmoglein 3 Ectodomain Induces Loss of Keratinocyte Adhesion, a Hallmark in Pemphigus Vulgaris.
Invest Dermatol., 143(2):254-263.
Egu DT, Schmitt T, Waschke J (2022)
Mechanisms Causing Acantholysis in Pemphigus-Lessons from Human Skin.
Front Immunol. 13:884067. PMID: 35720332 Free PMC article. Review.
Schmitt T, Egu DT, Walter E, Sigmund AM, Eichkorn R, Yazdi A, Schmidt E, Sárdy M, Eming R, Goebeler M, Waschke J (2021)
Ca2+ signalling is critical for autoantibody-induced blistering of human epidermis in pemphigus.
Br J Dermatol. 185(3):595-604. doi: 10.1111/bjd.20091. PMID: 33792909
Egu DT, Sigmund AM, Schmidt E., Spindler V, Walter E, Waschke J (2020)
A new ex vivo human oral mucosa model reveals that p38MAPK inhibition is not effective to prevent autoantibody-induced mucosal blistering in pemphigus.
Br J Dermatol, 182(4):987-994 .
Fuchs M, Sigmund AM, Waschke J, Vielmuth F. (2020)
Desmosomal Hyperadhesion Is Accompanied with Enhanced Binding Strength of Desmoglein 3 Molecules.
Biophys J. 119(8):1489-1500. PMID: 33031738 Free PMC article.
Sigmund AM, Steinert LS, Egu DT, Bayerbach FC, Waschke J, Vielmuth F (2020)
Dsg2 Upregulation as a Rescue Mechanism in Pemphigus.
Front Immunol. 28;11:581370
Meier K, Holstein J, Solimani F, Waschke J, Ghoreschi K (2020)
Case Report: Apremilast for Therapy-Resistant Pemphigus Vulgaris.
Front Immunol. 11:588315.
Schmitt T, Waschke J (2020)
Autoantibody-Specific Signalling in Pemphigus.
Front Med, 9;8:701809. PMID: 34434944 Free PMC article. Review.
Radeva MY, Walter E, Stach RA, Yazdi AS, Schlegel N, Sarig O, Sprecher E, Waschke J (2019)
ST18 Enhances PV-IgG-Induced Loss of Keratinocyte Cohesion in Parallel to Increased ERK Activation.
Front Immunol. 2019 Apr 17;10:770. doi: 10.3389/fimmu.2019.00770. eCollection 2019.
Kugelmann D, Rötzer V, Walter E, Egu DT, Fuchs MT, Vielmuth F, Vargas-Robles H, Schnoor M, Hertl M, Eming R, Rottner K, Schmidt A, Spindler V, Waschke J (2019)
Role of Src and Cortactin in Pemphigus Skin Blistering.
Front Immunol. 2019 Apr 4;10:626. doi: 10.3389/fimmu.2019.00626. eCollection 2019.
Kugelmann D, Radeva MY, Spindler V, Waschke J (2019)
Dsg1 deficiency causes lethal skin blistering.
J Invest Dermatol. 2019 Jan 17. pii: S0022-202X(19)30017-X. doi: 10.1016/j.jid.2019.01.002.
Egu DT, Kugelmann D, Waschke J (2019)
Role of PKC and ERK Signaling in Epidermal Blistering and Desmosome Regulation in Pemphigus.
Front Immunol. 2019 Dec 6;10:2883. doi: 10.3389/fimmu.2019.02883. eCollection 2019.
Walter E, Vielmuth F, Rotkopf L, Sárdy M, Horváth ON, Goebeler M, Schmidt E, Eming R, Hertl M, Spindler V, Waschke J (2017)
Different signaling patterns contribute to loss of keratinocyte cohesion dependent on autoantibody profile in pemphigus.
Sci Rep. (2017) 7:3579. doi: 10.1038/s41598-017-03697-7.
Egu DT, Walter E, Spindler V, Waschke J (2017)
Inhibition of p38MAPK signaling prevents epidermal blistering and alterations of desmosome structure induced by pemphigus autoantibodies in human epidermis.
Br J Dermatol. (2017) 177:1612–8. doi: 10.1111/bjd.15721.
Rötzer V, Hartlieb E, Winkler J, Walter E, Schlipp A, Sardy M, Spindler V, Waschke J (2016)
Desmoglein 3-Dependent Signaling Regulates Keratinocyte Migration and Wound Healing.
J Invest Dermatol. 2016 Jan;136(1):301-10. doi: 10.1038/JID.2015.380.
Rötzer V, Hartlieb E, Vielmuth F, Gliem M, Spindler V, Waschke J (2015)
E-cadherin and Src associate with extradesmosomal Dsg3 and modulate desmosome assembly and adhesion.
Cell Mol Life Sci. 2015 Dec;72(24):4885-97. doi: 10.1007/s00018-015-1977-0. Epub 2015 Jun 27.
Vielmuth F, Waschke J, Spindler V (2015)
Loss of Desmoglein Binding Is Not Sufficient for Keratinocyte Dissociation in Pemphigus.
J Invest Dermatol. 2015 Dec;135(12):3068-3077. doi: 10.1038/jid.2015.324. Epub 2015 Aug 19.
Spindler V, Dehner C, Hübner S, Waschke J (2014)
Plakoglobin but not desmoplakin regulates keratinoycte cohesion via p38MAPK signaling.
J Invest Dermatol. 2014, 134(6):1655-64
Rötzer V, Breit A, Waschke J, Spindler V (2014)
Adducin is required for keratinocyte cohesion.
J Biol Chem. 2014, 289(21):14925-40
Hartlieb E, Rötzer V, Radeva M, Spindler V, Waschke J (2014)
Desmoglein 2 compensates for desmoglein 3 but does not control cell adhesion via regulation of p38-mitogen-activated protein-kinase in keratinocytes.
J Biol Chem. 2014, 289(24):17043-17053
Dehner C, Rötzer V, Waschke J, Spindler V (2014)
A desmoplakin point mutation with enhanced keratin association ameliorates pemphigus vulgaris autoantibody-mediated loss of cell cohesion.
Am J Pathol. 2014, 184(9):2528-36.
Hartlieb E, Kempf B, Partilla M, Vigh B, Spindler V, Waschke J (2013)
Desmoglein 2 is less important than desmoglein 3 for keratinocyte cohesion.
PLoS One. 2013;8(1):e53739. doi: 10.1371/journal.pone.0053739. Epub 2013 Jan 11.
Spindler V, Rötzer V, Dehner C, Kempf B, Gliem M, Radeva M, Hartlieb E, Harms GS, Schmidt E, Waschke J (2013)
Peptide-mediated desmoglein 3 crosslinking prevents pemphigus vulgaris autoantibody-induced skin blistering.
J Clin Invest. 2013, 123(2):800–811

