Biophysical-characterization-of-cell-cohesion
Members:
Franziska Vielmuth (group leader)
Michael Fuchs (PhD student)
Letyfee Steinert (PhD student)
Desmosomal cadherins provide strong intercellular adhesion and thus are most abundant in tissues constantly exposed to mechanical stress, such as the heart and the epidermis. Dysfunction of desmosomal cadherin interaction lead to severe diseases such as pemphigus (in the skin and mucous membranes) and arrhythmogenic cardiomyopathy. Therefore, we investigate the structure and binding properties of desmosomal cadherins to understand their regulation and dysfunction in the respective diseases.
To do so, we use a set of biophysical and imaging techniques, which allow characterization of desmosomal cadherin distribution, mobility and binding properties. Especially, atomic force microscopy (AFM) is a central technique in this project and can be combined with several other approaches such as conventional fluorescence microscopy and STED. AFM is a laser-based technique in which a sharp tip on a flexible cantilever is repetitively lower to and retracted from a probe surface. The deflection of the tip while contacting the surface is detected by a laser which is pointed onto it (Figure 1, left panel). From these measurements, information about the surface topography and mechanical properties (such as Young’s modulus) of living cells under near-physiological conditions can be gained at nanometer scale. Further, by functionalizing the scanning tip with recombinant adhesion proteins (e.g. Desmoglein (Dsg) 3) we can detect specific single molecule interaction and characterize their distribution, mobility and binding properties on the respective cells (Fig. 1, right panel). In another biophysical approach, we use optical tweezers which allow the investigation of cell surface-attached microbeads that were functionalized with specific adhesion molecules.
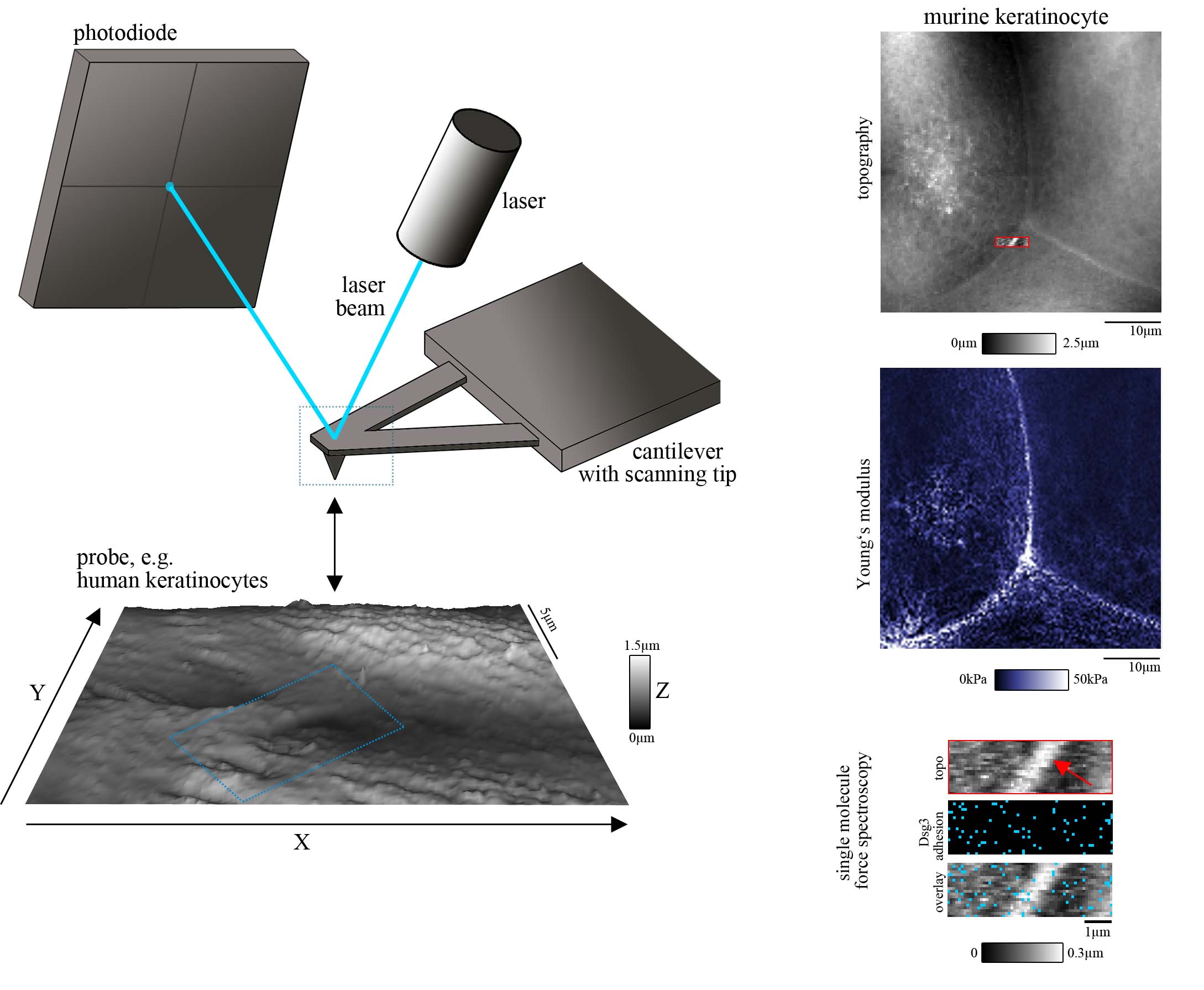
Figure 1: AFM setup (left panel), AFM measurement including topography, mechanical properties (such as elasticity as determined by the Young’s Modulus) and single molecule force spectroscopy (for Dsg3) on living murine keratinocytes (right panel).
Figure modified from Vielmuth et al, Minireview in Front in Immunol, 2018.
Applying these techniques, we gained insight into the function of cell adhesion molecules in cellular context. We observed that Dsg binding properties depend on intracellular desmosomal proteins such as keratins and Plakophilins as well as on signaling molecules such as p38 mitogen-activated protein kinase (p38MAPK) and proteinkinase C (PKC). Those proteins regulate the adhesive strength and membrane availability as well as distribution and mobility of the molecules in the cell membrane.
In the context of the bullous autoimmune disease pemphigus in which autoantibodies directed against the desmosomal adhesion molecules Dsg1 and 3 lead loss of keratinocyte cohesion which is clinically represented by flaccid blisters of the epidermis and mucous membranes (pemphigus) we investigated the interference of pathogenic autoantibodies with Dsg1 or 3 interactions, respectively. Interestingly, direct inhibition could only be observed for Dsg3, but not for Dsg1. However, in both cases a keratin- and p38MAPK-dependent redistribution of the molecules along the cell borders were detected using AFM which contributes to loss of intercellular adhesion (Figure 2).
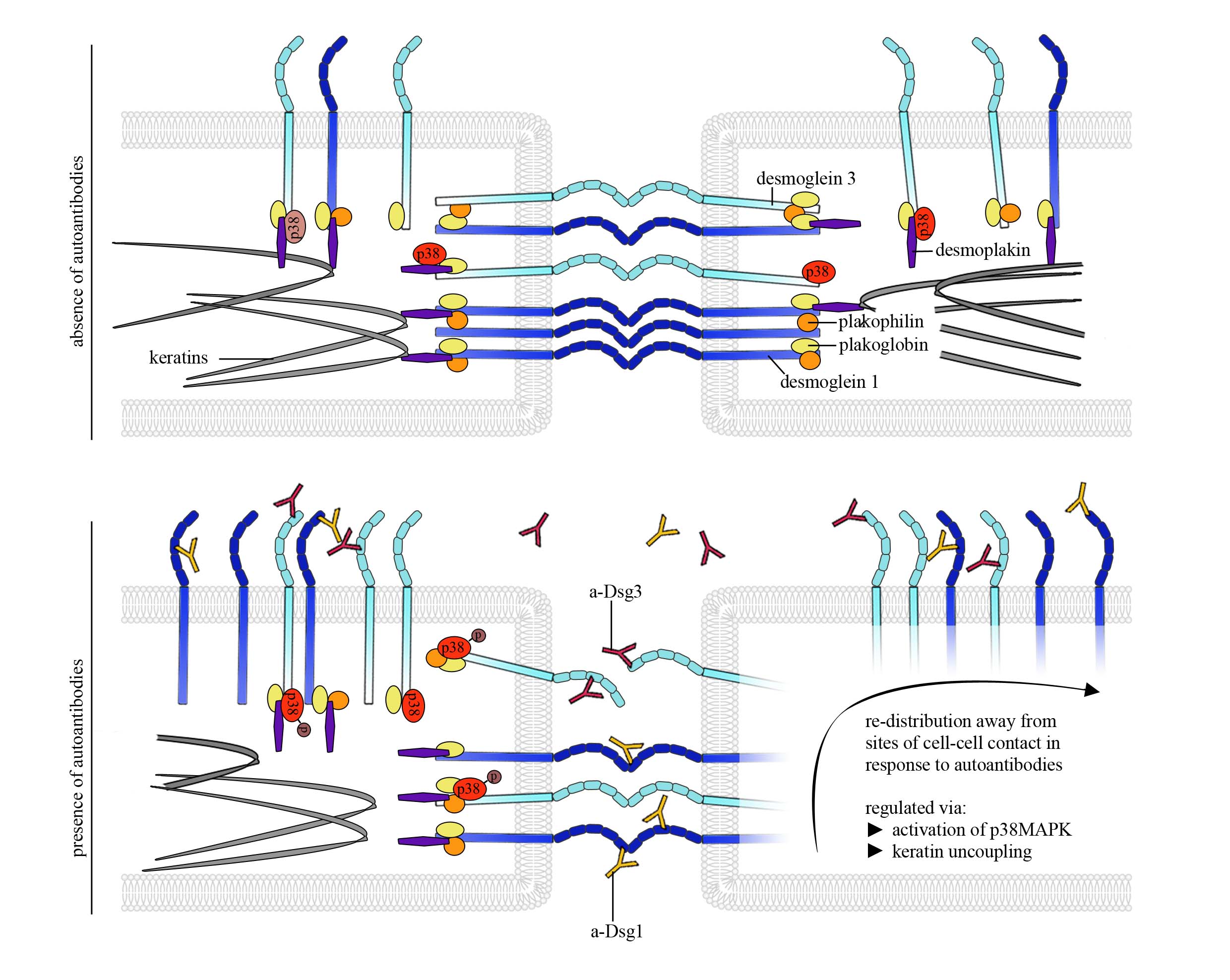
Figure 2: AFM provides insights into molecule re-distribution and changes in Dsg binding properties in pemphigus pathogenesis
Figure modified from Vielmuth et al, Minireview in Front in Immunol, 2018.
Taken together, biophysical techniques are powerful tools in adhesion research and can contribute to a better understanding of regulation of desmosomal adhesion in various tissues. We aim to get better insights into single molecule interaction mechanisms as a basic to created new strategies how compromised desmosomal adhesion can be restored and may develop new therapeutic strategies in desmosome-based diseases.
Recent publications (2013 to present):
Sigmund AM, Egu DT, Bayerbach FC, Waschke J, Vielmuth F. Dsg2 Upregulation as a Rescue Mechanism in Pemphigus. Front Immunol. 2020
Fuchs M, Sigmund AM, Waschke J, Vielmuth F. Desmosomal Hyperadhesion Is Accompanied with Enhanced Binding Strength of Desmoglein 3 Molecules. Biophys J. 2020 Sep 21:S0006-3495(20)30718-9. doi: 10.1016/j.bpj.2020.09.008. Online ahead of print.
Schinner C, Olivares-Florez S, Schlipp A, Trenz S, Feinendegen M, Flaswinkel H, Kempf E, Egu DT, Yeruva S, Waschke J The inotropic agent digitoxin strengthens desmosomal adhesion in cardiac myocytes in an ERK1/2-dependent manner Basic Res Cardiol . 2020 Jun 17;115(4):46. doi: 10.1007/s00395-020-0805-3.
Fuchs M, Foresti M, Radeva MY, Kugelmann D, Keil R, Hatzfeld M, Spindler V, Waschke J, Vielmuth F. Plakophilin 1 but not plakophilin 3 regulates desmoglein clustering. Cell Mol Life Sci. 2019 Apr 4. doi: 10.1007/s00018-019-03083-8.
Vielmuth F, Spindler V, Waschke J. (2018) Atomic Force Microscopy Provides New Mechanistic Insights into the Pathogenesis of Pemphigus. Front Immunol. 2018 Mar 28;9:485. doi: 10.3389/fimmu.2018.00485. eCollection 2018. Review.
Vielmuth F, Walter E, Fuchs M, Radeva MY, Buechau F, Magin TM, Spindler V, Waschke J. (2018) Keratins Regulate p38MAPK-Dependent Desmoglein Binding Properties in Pemphigus.Front Immunol. 2018 Mar 19;9:528. doi: 10.3389/fimmu.2018.00528. eCollection 2018.
Vielmuth F, Wanuske MT, Radeva MY, Hiermaier M, Kugelmann D, Walter E, Buechau F, Magin TM, Waschke J, Spindler V. (2018) Keratins Regulate the Adhesive Properties of Desmosomal Cadherins through Signaling. J Invest Dermatol. 2018 Jan;138(1):121-131. doi: 10.1016/j.jid.2017.08.033. Epub 2017 Sep 9.
Ungewiß H, Vielmuth F, Suzuki ST, Maiser A, Harz H, Leonhardt H, Kugelmann D, Schlegel N, Waschke J. Desmoglein 2 regulates the intestinal epithelial barrier via p38 mitogen-activated protein kinase. Sci Rep. 2017 Jul 24;7(1):6329. doi: 10.1038/s41598-017-06713-y.
Schinner C, Vielmuth F, Rötzer V, Hiermaier M, Radeva MY, Co TK, Hartlieb E, Schmidt A, Imhof A, Messoudi A, Horn A, Schlipp A, Spindler V, Waschke J. (2017) Adrenergic Signaling Strengthens Cardiac Myocyte Cohesion. Circ Res. 2017 Apr 14;120(8):1305-1317. doi: 10.1161/CIRCRESAHA.116.309631. Epub 2017 Mar 13.
Vielmuth F, Schumann RG, Spindler V, Wolf A, Scheler R, Mayer WJ, Henrich PB, Haritoglou C. (2016) Biomechanical Properties of the Internal Limiting Membrane after Intravitreal Ocriplasmin Treatment. Ophthalmologica. 2016;235(4):233-40. doi: 10.1159/000444508. Epub 2016 Apr 28.
Vielmuth F, Waschke J, Spindler V. (2015) Loss of Desmoglein Binding Is Not Sufficient for Keratinocyte Dissociation in Pemphigus. J Invest Dermatol. 2015 Dec;135(12):3068-3077. doi: 10.1038/jid.2015.324. Epub 2015 Aug 19.
Spindler V, Meir M, Vigh B, Flemming S, Hütz K, Germer CT, Waschke J, Schlegel N (2015). Loss of desmoglein2 contributes to the pathogenesis of Crohn’s disease. Inflamm Bowel Dis, in press
Flemming S, Burkard N, Renschler M, Vielmuth F, Meir M, Schick MA, Wunder C, Germer CT, Spindler V, Waschke J, Schlegel N (2015). Soluble VE-cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis. Cardiovasc Res, ePub: DOI: 10.1093/cvr/cvv144
Vielmuth F, Hartlieb E, Kugelmann D, Waschke J, Spindler V (2014); Atomic force microscopy identifies regions of distinct desmoglein 3 adhesive properties on living keratinocytes; Nanomedicine, 11(3):511-20
Monteiro AC, Luissint AC, Sumagin R, Lai C, Vielmuth F, Wolf MF, Laur O, Reiss K, Spindler V, Stehle T, Dermody TS, Nusrat A, Parkos CA (2014). Trans-dimerization of JAM-A Regulates Rap2 and is Mediated by a Domain that is Distinct from the Cis-dimerization Interface; Mol Biol Cell, 25(10):1574-85
Spindler V, Rötzer V, Dehner C, Kempf B, Gliem M, Radeva M, Hartlieb E, Harms GS, Schmidt E, Waschke J (2013). Peptide-mediated desmoglein 3 crosslinking prevents pemphigus vulgaris autoantibody-induced skin blistering; J Clin Invest, 123(2):800–811
Hartlieb E, Partilla M, Vigh B, Spindler V, Waschke J (2013). Desmoglein 2 is less important than Dsg3 for keratinocyte cohesion PLOS One, 8(1): e53739

