Endothelial barrier dysfunction and inflammation
Members:
Mariya Y. Radeva
Alexander Garcia Ponce
Sina Moztarzadeh (PhD student)
Ibrahim Hamad (MD student)
Under allergic and inflammatory conditions, the functional integrity of the endothelium lining the inner surface of the blood vessels is compromised primarily by intercellular gap formation. This effect is associated with reduced endothelial barrier function and increased paracellular permeability.
Modulation of endothelial barrier function can be achieved by number of molecules such as the second messenger cyclic adenosine monophosphate (cAMP), members of the Rho family of small GTPases, i.e RhoA and Rac1, as well as actin-binding proteins (ABPs).
Our previous studies demonstrated that cAMP-mediated activation of the small GTPase Rac1 is an essential signaling step required for stabilization of endothelial barrier function. In this respect, it was shown that Rac1 is activated by both the exchange protein directly activated by cAMP (Epac1)/ Ras-related protein 1 (Rap1) and protein kinase A (PKA)-dependent signaling pathways (Figure 1).
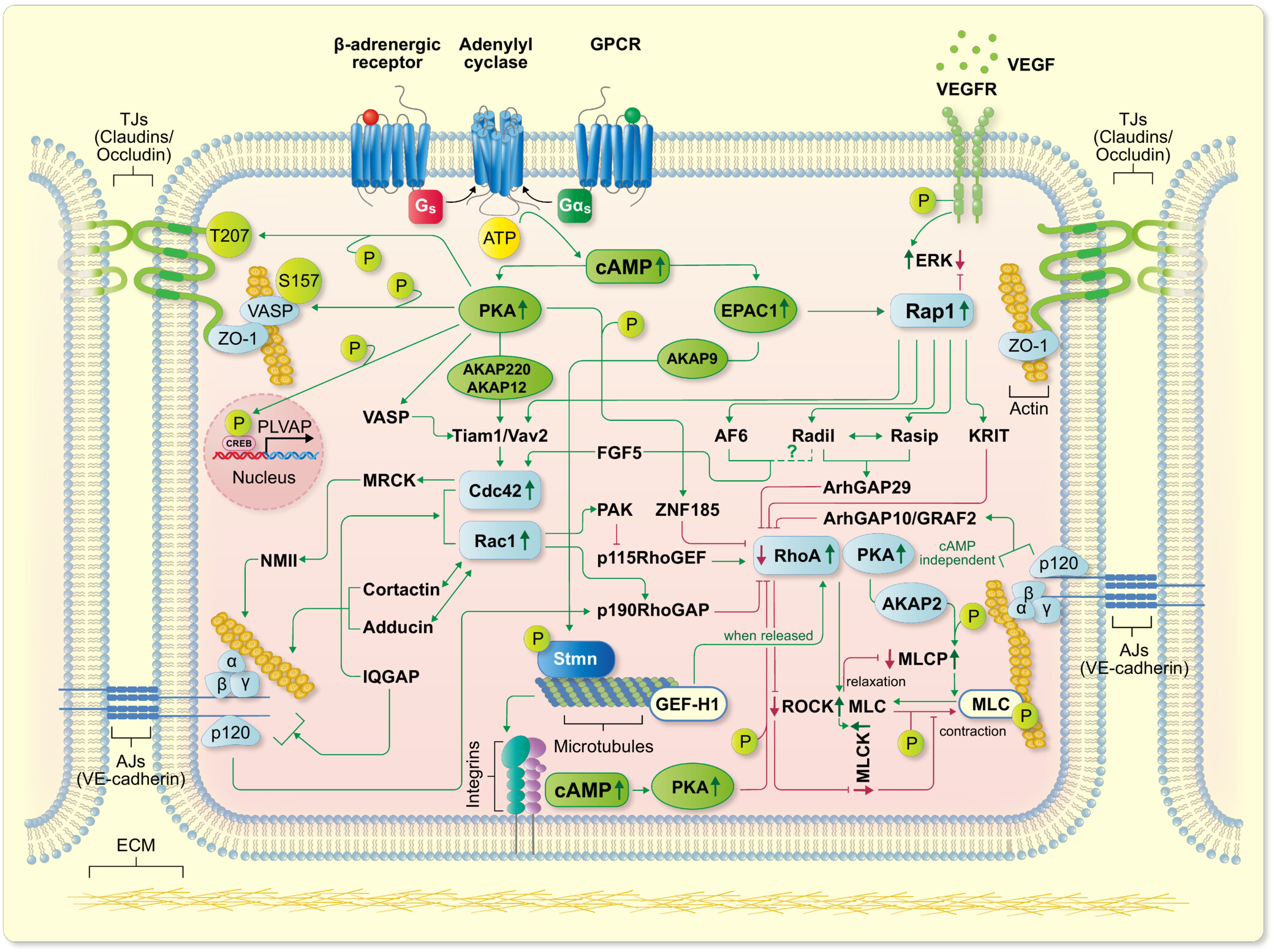
Figure 1. Mechanisms involved in cAMP- mediated regulation of cadherin adhesion and barrier function in the endothelium. cAMP produced under control of β- adrenergic signaling activates PKA and Epac1 which control the activity of Rho family GTPases and thereby fine- tune the balance of cytoskeletal anchorage and actin– myosin contraction to regulate endothelial AJ; Vielmuth F et al, Acta Physiol (Oxf). 2023
On another side, tight regulation of PKA function can be achieved by discrete compartmentalization of the enzyme via physical interaction with A-kinase anchoring proteins (AKAPs). Apparently, this signaling pathway is impaired in inflammation and sepsis, since the levels of cAMP become lower and Rac1 is inactivated, contributing to endothelial barrier breakdown. Therefore, our group worked on discovering the functional relevance of AKAP molecules for maintenance of endothelial barrier integrity and cytoskeleton reorganization. So far we found AKAP220 and AKAP12 as new candidates relevant for maintenance of endothelial barrier integrity (Figure 1), Radeva MY et al.; PLoS One, 2014 .
In addition, we studied the role of Epac1-dependent pathways in the maintenance of endothelial barrier. These investigations were designed as follow-up research in Epac1-knockout mice that increased the basal permeability to high and low molecular weight tracers in different microvascular beds such as in the skin, small and large intestine, and adipose tissue.
Epac1 is a central player working through multiple mechanisms to promote cAMP-mediated endothelial barrier stabilization, including modulation of junctional integrity and rearrangement of cortical actin as well as by cross-talk between GTPases. In this respect, our recent study emphasize that part of the Epac1 effect might be mediated by regulation of not only Rap1, but also by simultaneous involvement of Rac1 and RhoA molecules, García-Ponce A et al.; Cells. 2020
Furthermore, the control of actin dynamics is crucial for endothelial barrier stability in vivo, since strengthening of the cortical actin cytoskeleton, partially regulated by actin-binding proteins (ABPs), controls the dynamics of junctional proteins and thus endothelial barrier integrity. In line with this, a growing number of ABPs such as vasodilator-stimulated phosphoprotein (VASP), cortactin (CTTN) and adducin (ADD) were shown to play a significant role in modulation of endothelial barrier (Figure 1).
The interaction of ABPs with the actin cytoskeleton is diverse. In this respect, it was shown that CTTN contributes to branching of pre-existent actin filaments necessary for different cellular functions. On the other hand, ADD is a key component for the assembly of actin-spectrin networks important for supporting plasma membrane. Although, the ABPs associations with the actin cytoskeleton have been extensively studied, their relation with intercellular contacts and thus, endothelial barrier homeostasis is still not completely understood. For this reason, our group is interested in elucidating the role of CTTN and ADD in the maintenance of cAMP-mediated endothelial barrier integrity.
Our recent research showed that the lack of both, CTTN and ADD, led to compromised barrier integrity, and resulted in fragmented distribution of different junctional proteins. Moreover, neither ADD nor CTTN-KO cells respond to elevated intercellular concentration of cAMP typically characterized with enhanced barrier function as well as junctional and actin cytoskeleton reorganization. Our data also showed that CTTN is required for cAMP-mediated activation of Rap1/Rac1 signaling pathway as well as that ADD may possibly acts upstream from Rac1 and is necessary for its activation via cAMP, Kugelmann D et al.; PLoS One 2015; Moztarzadeh S et al.; Sci Rep. 2022.
Another topic our group is focused on is the role of γ-catenin or more commonly known as plakoglobin (PG) in endothelial barrier integrity. PG is a structural and functional homologue of ß-catenin and therefore, in a similar fashion to the latter, exerts a dual function of being structural/ cell adhesion related and signaling molecule, capable of translocating to the nucleus and modulating cell transcription or protein stability in both a ß-catenin- dependent and – independent manner. Recent studies have shown the important role of PG in the regulation of endothelia barrier integrity. However, the relationship between PG and cAMP- mediated endothelial barrier stabilization is not clear yet. In this respect we could verify that lack of PG modulates endothelial barrier integrity. The effect was accompanied with accumulation of more VE-cadherin and ß-catenin towards the cell contacts. Interestingly, the presence of PG also induced translocation of CTTN to the junctions. In addition, cAMP elevation mediates barrier tightening in both WT and PG-KO cells, but this effect was less prominent in PG-KO cells. cAMP-mediated recruitment of junctional and junctional-related proteins towards the junctions was unaffected due to PG-KO. We also could demonstrate that cAMP mediated Rac1 activation was independent on PG.
As part of our experimental procedures, we employ in vitro cellular models and measure Transendothelial Electrical Resistance (TER) from different cell monolayers. These analyses are combined with structural, pharmacological and biochemical investigations. Moreover, atomic force microscopy and optical laser tweezer are readily available to study junctional proteins binding forces.
Publications:
Hamad I, Sepic S, Moztarzadeh S, García-Ponce A, Waschke J, Radeva MY (2025)
Plakoglobin does not participate in endothelial barrier stabilization mediated by cAMP.
Sci Rep.;15(1):9043. doi: 10.1038/s41598-025-93756-1.
Moztarzadeh S, Vargas-Robles H, Schnoor M, Radeva MY, Waschke J, Garcia-Ponce A (2024)
Erk1/2 is not required for endothelial barrier establishment despite its requirement in cAMP-dependent Rac1 activation.
Tissue Barriers, 2398875. doi: 10.1080/21688370.2024.2398875.
Moztarzadeh S, Sepic S, Hamad I, Waschke J, Radeva MY, García-Ponce A (2024)
Cortactin is in a complex with VE-cadherin and is required for endothelial adherens junction stability through Rap1/Rac1 activation.
Sci Rep. 14(1):1218.
Knop JL, Burkard N, Danesh M, Kintrup S, Dandekar T, Srivastava M, Springer R, Hiermaier M, Wagner NM, Waschke J, Flemming S, Schlegel N. (2023)
Endothelial barrier dysfunction in systemic inflammation is mediated by soluble VE-cadherin interfering VE-PTP signaling.
iScience. 26(10):108049.
Moztarzadeh S, Radeva MY, Sepic S, Schuster K, Hamad I, Waschke J, García-Ponce A (2022)
Lack of adducin impairs the stability of endothelial adherens and tight junctions and may be required for cAMP-Rac1-mediated endothelial barrier stabilization.
Sci Rep. 2022.; Sep 2;12(1):14940. DOI: 10.1038/s41598-022-18964-5
García-Ponce A, Schuster K, Døskeland SO, Reed RK, Curry FE, Waschke J, Radeva MY (2020)
Epac1 Is Crucial for Maintenance of Endothelial Barrier Function through A Mechanism Partly Independent of Rac1.
Cells. 2020.; Sep 25; 9(10):E2170. DOI: 10.3390/cells9102170.PMID: 32992982
Kugelmann D, Rotkopf LT, Radeva MY, Garcia-Ponce A, Walter E, Waschke J (2018)
Histamine causes endothelial barrier disruption via Ca2+-mediated RhoA activation and tension at adherens junctions.
Sci Rep. 2018.; Sep 5;8(1):13229. DOI: 10.1038/s41598-018-31408-3
Kugelmann D, Waschke J, Radeva MY (2015)
Adducin is involved in endothelial barrier stabilization.
PLoS One 2015.; 10(5). DOI: 10.1371/journal.pone.0126213
Flemming S, Burkard N, Renschler M, Vielmuth F, Meir M, Schick MA, Wunder C, Germer CT, Spindler V, Waschke J, Schlegel N (2015)
Soluble VE-cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis.
Cardiovasc Res. 2015.; 107(1):32-44. DOI: 10.1093/cvr/cvv144
Adamson RH, Clark JF, Radeva M, Kheirolomoom A, Ferrara KW, Curry FE. (2014)
Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect.
Am J Physiol Heart Circ Physiol. 2014.; Apr 1;306(7):H1011-7. DOI: 10.1152/ajpheart.00829.2013
Radeva MY, Kugelmann D, Spindler V, Waschke J (2014)
PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation.
PLoS One. 2014.; 9(9). DOI: 10.1371/journal.pone.0106733
Schlegel N, Leweke R, Meir M., Germer CT, Waschke J (2012)
Role of NF-κB activation in LPS-induced endothelial barrier breakdown. Histochem.
Cell Biol. 2012.; 138(4):627-641. DOI: 10.1007/s00418-012-0983-7
Schick MA, Wunder C, Wollborn J, Roewer N, Waschke J, Germer CT, Schlegel N (2012)
Phosphodiesterase-4 inhibition as a therapeutic approach to treat capillary leakage in systemic inflammation
J. Physiol. 2012.; 590(Pt 11):2693-2708. DOI: 10.1113/jphysiol.2012.232116
Chen W, Gaßner B, Börner S, Nikolaev VO, Schlegel N, Waschke J, Steinbronn N, Strasser R, Kuhn M (2012)
Atrial natriuretic peptide enhances microvascular albumin permeability by the caveolae-mediated transcellular pathway.
Cardiovasc Res. 2012.; 93(1): 141-151. DOI: 10.1093/cvr/cvr279
Spindler V, Peter D, Harms GS, Asan E, Waschke J (2011)
Ultrastructural analysis reveals cAMP-dependent enhancement of microvascular endothelial barrier functions via Rac1-mediated reorganization of intercellular junctions.
Am J Pathol. 2011.; 178(5):2424-2436. DOI: 10.1016/j.ajpath.2011.01.014
Spindler V, Waschke J (2011)
Beta-adrenergic stimulation contributes to maintenance of endothelial functions under baseline conditions.
Microcirculation, 2011.; 18(2): 118-127. DOI: 10.1111/j.1549-8719.2010.00072.x
Chtcheglova L, Wildling L, Waschke J, Drenckhahn D, Hinterdorfer P (2010)
AFM functional imaging on vascular endothelial cells.
J.Mol.Recognit. 2010.; 23(6): 598-596. DOI: 10.1002/jmr.1052
Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Münzel T, Renné T (2010)
Differential VASP phosphorylation controls remodeling of the actin cytoskeleton.
J. Cell Sci. 2010.; 122:3954-3965. DOI: 10.1242/jcs.044537
Schick MA, Isbary TJ, Schlegel N, Brugger J, Waschke J, Muellenbach R, Roewer N, Wunder C (2010)
The impact of crystalloid infusion on the kidney in rodent sepsis.
Intensive Care Med. 2010.; 36(3). 541-548. DOI: 10.1007/s00134-009-1704-0
Schlegel N, Waschke J (2009)
Impaired cAMP and Rac 1 signalling contribute to TNF-α-induced endothelial barrier breakdown in microvascular endothelium.
Microcirculation, 2009.; 16: 521-533. DOI: 10.1080/10739680902967427
Baumer Y, Spindler V, Werthmann R, Buenemann M, Waschke J (2009)
Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown.
J. Cell Physiol. 2009.; 220: 716-726. DOI: 10.1002/jcp.21819
Schlegel N, Waschke J (2009)
Impaired integrin-mediated adhesion contributes to reduced barrier properties in VASP-deficient microvascular endothelium.
J. Cell. Physiol, 2009.; 220:357-366. DOI: 10.1002/jcp.21772
Schlegel N, Baumer Y, Drenckhahn D, Waschke J (2009)
LPS-induced endothelial barrier breakdown is cAMP-dependent in vivo and in vitro. Crit.
Care Med. 2009.; 37(5): 1735-43. DOI: 10.1097/CCM.0b013e31819deb6a
Schlegel N, Waschke J (2009)
VASP is involved in cAMP-mediated Rac 1 activation in microvascular endothelial cells.
AJP Cell Physiology, 2009.; 296(3): C453-462. DOI: 10.1152/ajpcell.00360.2008
Samarin J, Rehm M, Krueger B, Waschke J, Goppelt-Struebe M (2009)
Up-regulation of connective tissue growth factor in endothelial cells by the microtubule-destabilizing agent combretastatin A-4. Mol.
Cancer Res. 2009.; 7(2): 180-188. DOI: 10.1158/1541-7786.MCR-08-0292
Baumer Y, Drenckhahn D, Waschke J (2008)
cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem.
Cell Biol. 2008.; 129(6): 765-778. DOI: 10.1007/s00418-008-0422-y
Schlegel N, Burger S, Golenhofen N, Walter U, Drenckhahn D, Waschke J (2008)
The role of VASP in the regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization.
Am. J. Physiol. Cell Physiol 2008.; 294(1): c178-188. DOI:10.1152/ajpcell.00273.2007.
Baumer Y, Burger S, Curry FE, Golenhofen N, Drenckhahn D, Waschke J (2008)
Different role of Rho GTPases in endothelial barrier regulation dependent on endothelial cell origin. Histochem.
Cell Biol. 2008.; 129(2): 179-191. DOI: 10.1002/jcp.22446

